Organic Chemistry Text Book (CHEM 3401 and 3402)
- Home
- Chapter 1: A Review of General ChemistryToggle Dropdown
- 1.1 Introduction to Organic Chemistry
- 1.2 Electrons, Bonds, and Lewis Structures
- 1.3 Identifying Formal Charges
- 1.4 Atomic Orbitals
- 1.5 Valence Bond Theory
- 1.6 Molecular Orbital Theory/Hybridization
- 1.7 VSEPR Theory: Predicting Geometry
- 1.8 Dipole Moments and Molecular Polarity
- 1.9 Intermolecular Forces and Physical Properties
- Problem Set
- Videos for chapter 1
- Chapter 2: Molecular RepresentationsToggle Dropdown
- Chapter 3: Acids and BasesToggle Dropdown
- Chapter 4: Alkanes and CycloalkanesToggle Dropdown
- Chapter 5: StereochemistryToggle Dropdown
- Chapter 6: Chemical Reactivity and MechanismsToggle Dropdown
- Chapter 7: Substitution ReactionsToggle Dropdown
- Chapter 8: Addition Reactions of AlkenesToggle Dropdown
- 8.1 Introduction of Addition Reactions
- 8.1 Nomenclature of Alkenes
- 8.2 Hydrohalogenation of Alkenes
- 8.3 Hydration, Hydroboration, and Oxymercuration of Alkenes
- 8.4 Hydrogenation of Alkenes
- 8.5 Halogenation of Alkenes
- 8.6 Dihydroxylation, Epoxidation, and Ozonolysis of Alkenes
- Problem Set
- Chapter 8 Videos
- Chapter 9: AlkynesToggle Dropdown
- Chapter 10: Radicals
- Chapter 11: SynthesisToggle Dropdown
- Problem Sets Organic Chemistry I (CHEM 3401)
- Chapter 12: Alcohols and PhenolsToggle Dropdown
- 12.1 Alcohol Structure
- 12.2 Solubility
- 12.3 Boiling Point & Melting Point
- 12.4 Nomenclature
- 12.5 Alcohol Acidity
- 12.6 Reactions of Alcohols and Phenols
- 12.6.1 Substitution of the Hydroxyl Hydrogen
- 12.6.2 Nucleophilic Substitution of the Hydroxyl Group
- 12.6.3 Elimination Reactions of Alcohols
- 12.6.4 Oxidation Reactions of Alcohols
- 12.6.5 Reactions of Phenols
- 12.7 Practice Problems
- 12.7.1 Alcohol Nomenclature 1
- 12.7.2 Alcohol Nomenclature 2
- 12.7.3 Alcohol Nomenclature 3
- 12.7.4 Formation of Carbonyl Compounds
- 12.7.5 Functional Relationships of Alcohols
- 12.7.6 Reactions of Alcohols & Phenols
- 12.7.7 Alcohol Reactions
- Chapter 13: Ethers and EpoxidesToggle Dropdown
- Chapter 14: Infrared Spectroscopy and Mass SpectrometryToggle Dropdown
- 14.1 Introduction fo Molecular Spectroscopy
- 14.2 Infrared Spectroscopy
- 14.2.1 Introduction
- 14.2.2 Vibrational Spectroscopy
- 14.2.3 Group Frequencies
- 14.2.4 Table of Characteristic IR Frequencies
- 14.3 Mass Spectrometry
- 14.3.1 The Mass Spectrometer
- 14.3.2 Characteristics of Mass Spectra
- 14.3.3 Isotopes
- 14.3.4 Fragmentation Patterns
- 14.3.5 High Resolution Spectra
- 14.3.6 MS Practice Problems
- 14.3.6a Problem 1
- 14.3.6b Problem 2
- 14.3.6c Problem 3
- 14.3.6d Problem 4
- 14.3.6e Problem 5
- 14.3.6f Problem 6
- 14.3.6g Problem 7
- 14.3.6h Problem 8
- Chapter 15: Nuclear Magnetic Resonance Spectroscopy and UV-Visible SpectroscopyToggle Dropdown
- 15.1 Nuclear Magnetic Resonance Spectroscopy
- 15.1.1 Background
- 15.1.2 Proton NMR Spectroscopy
- 15.1.2a Introduction to Proton NMR Spectroscopy
- 15.1.2b Chemical Shift
- 15.1.2c Signal Strength
- 15.1.2d Hydroxyl Proton Exchange and the Influence of Hydrogen Bonding
- 15.1.2e Pi-Electron Functions
- 15.1.2f Solvent Effects
- 15.1.2g Spin-Spin Interactions
- 15.1.2h Examples
- 15.1.3 Carbon NMR Spectroscopy
- 15.1.4 NMR Practice Problems
- 15.1.4a Problem 1
- 15.1.4b Problem 2
- 15.1.4c Problem 3
- 15.1.4d Problem 4
- 15.1.4e Problem 5
- 15.1.4f Problem 6
- 15.1.4g Problem 7
- 15.1.4h Problem 8
- 15.1.4i Problem 9
- 15.1.4j Problem 10
- 15.1.5 Table of Proton NMR Shifts
- 15.1.6 Table of Carbon NMR Shifts
- 15.2 UV-Visible Spectroscopy
- 15.2.1 Background
- 15.2.2 The Electromagnetic Spectrum
- 15.2.3 UV-Visible Absorption Spectra
- 15.2.4 The Importance of Conjugation
- 15.3 Spectroscopy Practice Problems
- Chapter 16: Conjugated Pi Systems and Pericyclic ReactionsToggle Dropdown
- Chapter 17: Aromatic CompoundsToggle Dropdown
- 17.1 Aromaticity
- 17.1.1 Benzene
- 17.1.2 Fused Ring Compounds
- 17.1.3 Other Aromatic Compounds
- 17.1.4 Antiaromaticity
- 17.1.5 Practice Problems
- 17.1.5a Problem 1
- 17.1.5b Problem 2
- 17.2 Reactions of Substituent Groups
- 17.2.1 Oxidation of Alkyl Side-Chains
- 17.2.2 Bromination of Alkyl Side-Chains
- 17.2.3 Reduction of Nitro Groups
- Chapter 17 Videos
- Chapter 18: Aromatic Substitution ReactionsToggle Dropdown
- 18.1 Electrophilic Aromatic Substitution Reactions
- 18.2 Electrophilic Aromatic Substitution Mechanism
- 18.3 Electrophilic Aromatic Substitution Activation/Deactivation and Orientation
- 18.4 Electrophilic Substitution of Disubstituted Benzene Rings
- 18.5 Practice Problems
- 18.5.1 Problem 1
- 18.5.2 Problem 2
- 18.5.3 Problem 3
- 18.5.4 Problem 4
- 18.5.5 Problem 5
- 18.5.6 Problem 6
- 18.5.7 Problem 7
- Chapter 18 Videos
- Chapter 19: Aldehydes and KetonesToggle Dropdown
- 19.1 Nomenclature
- 19.2 Preparation of Aldehydes and Ketones
- 19.3 Properties of Aldehydes and Ketones
- 19.4 Reactions of Aldehydes and Ketones
- 19.4.1 Addition Reactions
- 19.4.1a Hydration
- 19.4.1b Acetal Formation
- 19.4.1c Imine Formation
- 19.4.1d Cyanohydrin Formation
- 19.4.1e Hydride Reduction
- 19.4.1f Addition of Organometallic Reagents
- 19.4.2 Reduction of Aldehydes and Ketones
- 19.4.2a Wolff-Kishner Reduction
- 19.4.2b Clemmensen Reduction
- 19.4.3 Oxidation of Aldehydes and Ketones
- 19.5 Practice Problems
- 19.5.1 Problem 1
- 19.5.2 Problem 2
- 19.5.3 Problem 3
- 19.5.4 Problem 4
- 19.5.5 Problem 5
- 19.5.6 Problem 6
- 19.5.7 Problem 7
- 19.5.8 Problem 8
- 19.5.9 Problem 9
- 19.5.10 Problem 10
- 19.5.11 Problem 11
- 19.5.12 Problem 12
- Chapter 20: Carboxylic Acids and Their DerivativesToggle Dropdown
- 20.1 Nomenclature
- 20.2 Physical Properties
- 20.3 Acidity
- 20.4 Preparation of Carboxylic Acids
- 20.5 Reactions of Carboxylic Acids
- 20.5.1 Salt Formation
- 20.5.2 Substitution of the Hydroxyl Hydrogen
- 20.5.3 Substitution of the Hydroxyl Group
- 20.5.4 Reduction
- 20.5.5 Oxidation
- 20.6 Practice Problems-Carboxylic Acids
- 20.6.1 Nomenclature Practice-1
- 20.6.2 Nomenclature Practice-2
- 20.6.3 Acidity
- 20.6.4 Reactions of Carboxylic Acids
- 20.7 Carboxylic Acid Derivatives
- 20.7.1 Related Derivatives
- 20.7.2 Nomenclature
- 20.7.3 Reactions
- 20.7.3a Acyl Substitution
- 20.7.3b Nitrile Hydrolysis
- 20.7.3c Reductions
- 20.7.3d Reactions with Organometallic Reagents
- 20.7.3e Dehydration of Amides
- 20.7.4 Practice Problems-Carboxylic Acid Derivatives
- 20.7.4a Nomenclature Practice-1
- 20.7.4b Nomenclature Practice-2
- 20.7.4c Carbonyl Compounds
- 20.8 Practice Problems
- 20.8.1 Problem 1
- 20.8.2 Problem 2
- 20.8.3 Problem 3
- 20.8.4 Problem 4
- 20.8.5 Problem 5
- 20.8.6 Problem 6
- Chapter 21: Alpha Carbon Chemistry: Enols and EnolatesToggle Dropdown
- 21.1 Reactions at the Alpha Carbon
- 21.2 Alpha Halogenation of Enols and Enolates
- 21.3 Aldol Reaction
- 21.4 Claisen Condensation
- 21.5 Alkylation at the Alpha Position
- 21.5.1 Enolate Alkylation
- 21.5.2 Dicarbonyl Alkylation
- 21.5.3 Decarboxylation Following Alkylation
- 21.5.4 Conjugate Reactions
- 21.5.4a Michael Reaction
- 21.5.4b Robinson Annulation
- 21.5.4c With Hydrides and Organometallics
- 21.6 Practice Problem
- 21.6.1 Problem 1
- Org Chem II - Problem Sets - Collection (CHEM 3402)
- Problem Set
10.2 Halogenation of Alkanes
Halogenation
Halogenation is the replacement of one or more hydrogen atoms in an organic compound by a halogen (fluorine, chlorine, bromine or iodine). Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in which a C-H bond is broken and a new C-X bond is formed. The chlorination of methane, shown below, provides a simple example of this reaction.
CH4 + Cl2 + energy ——> CH3Cl + HCl
Since only two covalent bonds are broken (C-H & Cl-Cl) and two covalent bonds are formed (C-Cl & H-Cl), this reaction seems to be an ideal case for mechanistic investigation and speculation. However, one complication is that all the hydrogen atoms of an alkane may undergo substitution, resulting in a mixture of products, as shown in the following unbalanced equation. The relative amounts of the various products depend on the proportion of the two reactants used. In the case of methane, a large excess of the hydrocarbon favors formation of methyl chloride as the chief product; whereas, an excess of chlorine favors formation of chloroform and carbon tetrachloride.
The following facts must be accomodated by any reasonable mechanism for the halogenation reaction.
1. The reactivity of the halogens decreases in the following order: F2 > Cl2 > Br2 > I2.
2. We shall confine our attention to chlorine and bromine, since fluorine is so explosively reactive it is difficult to control, and iodine is generally unreactive.
3. Chlorinations and brominations are normally exothermic.
4. Energy input in the form of heat or light is necessary to initiate these halogenations.
5. If light is used to initiate halogenation, thousands of molecules react for each photon of light absorbed.
6. Halogenation reactions may be conducted in either the gaseous or liquid phase.
7. In gas phase chlorinations the presence of oxygen (a radical trap) inhibits the reaction.
8. In liquid phase halogenations radical initiators such as peroxides facilitate the reaction.
The most plausible mechanism for halogenation is a chain reaction involving neutral intermediates such as free radicals or atoms. The weakest covalent bond in the reactants is the halogen-halogen bond (Cl-Cl = 58 kcal/mole; Br-Br = 46 kcal/mole) so the initiating step is the homolytic cleavage of this bond by heat or light, note that chlorine and bromine both absorb visible light (they are colored). A chain reaction mechanism for the chlorination of methane has been described.
Bromination of alkanes occurs by a similar mechanism, but is slower and more selective because a bromine atom is a less reactive hydrogen abstraction agent than a chlorine atom, as reflected by the higher bond energy of H-Cl than H-Br.
To see an animated model of the bromination free radical chain reaction
Selectivity
When alkanes larger than ethane are halogenated, isomeric products are formed. Thus chlorination of propane gives both 1-chloropropane and 2-chloropropane as mono-chlorinated products. Four constitutionally isomeric dichlorinated products are possible, and five constitutional isomers exist for the trichlorinated propanes. Can you write structural formulas for the four dichlorinated isomers?
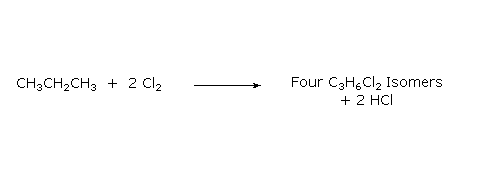 |
The halogenation of propane discloses an interesting feature of these reactions. All the hydrogens in a complex alkane do not exhibit equal reactivity. For example, propane has eight hydrogens, six of them being structurally equivalent primary, and the other two being secondary. If all these hydrogen atoms were equally reactive, halogenation should give a 3:1 ratio of 1-halopropane to 2-halopropane mono-halogenated products, reflecting the primary/secondary numbers. This is not what we observe. Light-induced gas phase chlorination at 25 ºC gives 45% 1-chloropropane and 55% 2-chloropropane.
CH3-CH2-CH3 + Cl2 ——> 45% CH3-CH2-CH2Cl + 55% CH3-CHCl-CH3
The results of bromination ( light-induced at 25 ºC ) are even more suprising, with 2-bromopropane accounting for 97% of the mono-bromo product.
CH3-CH2-CH3 + Br2 ——> 3% CH3-CH2-CH2Br + 97% CH3-CHBr-CH3
These results suggest strongly that 2º-hydrogens are inherently more reactive than 1º-hydrogens, by a factor of about 3:1. Further experiments showed that 3º-hydrogens are even more reactive toward halogen atoms. Thus, light-induced chlorination of 2-methylpropane gave predominantly (65%) 2-chloro-2-methylpropane, the substitution product of the sole 3º-hydrogen, despite the presence of nine 1º-hydrogens in the molecule.
(CH3)3CH + Cl2 ——> 65% (CH3)3CCl + 35% (CH3)2CHCH2Cl
If you are uncertain about the terms primary (1º), secondary (2º) & tertiary (3º) Click Here.
It should be clear from a review of the two steps that make up the free radical chain reaction for halogenation that the first step (hydrogen abstraction) is the product determining step. Once a carbon radical is formed, subsequent bonding to a halogen atom (in the second step) can only occur at the radical site. Consequently, an understanding of the preference for substitution at 2º and 3º-carbon atoms must come from an analysis of this first step.
First Step: R3CH + X· ——> R3C· + H-X
Second Step: R3C· + X2 ——> R3CX + X·
Since the H-X product is common to all possible reactions, differences in reactivity can only be attributed to differences in C-H bond dissociation energies. In our previous discussion of bond energy we assumed average values for all bonds of a given kind, but now we see that this is not strictly true. In the case of carbon-hydrogen bonds, there are significant differences, and the specific dissociation energies (energy required to break a bond homolytically) for various kinds of C-H bonds have been measured. These values are given in the following table.
| R (in R–H) | methyl | ethyl | i-propyl |
t-butyl |
phenyl | benzyl | allyl | vinyl |
|---|---|---|---|---|---|---|---|---|
| Bond Dissociation Energy (kcal/mole) |
103 | 98 | 95 | 93 | 110 | 85 | 88 | 112 |
The difference in C-H bond dissociation energy reported for primary (1º), secondary (2º) and tertiary (3º) sites agrees with the halogenation observations reported above, in that we would expect weaker bonds to be broken more easily than are strong bonds. By this reasoning we would expect benzylic and allylic sites to be exceptionally reactive in free radical halogenation, as experiments have shown. The methyl group of toluene, C6H5CH3, is readily chlorinated or brominated in the presence of free radical initiators (usually peroxides), and ethylbenzene is similarly chlorinated at the benzylic location exclusively. The hydrogens bonded to the aromatic ring (referred to as phenyl hydrogens above) have relatively high bond dissociation energies and are not substituted.
Since carbon-carbon double bonds add chlorine and bromine rapidly in liquid phase solutions, free radical substitution reactions of alkenes by these halogens must be carried out in the gas phase, or by other halogenating reagents. One such reagent is N-bromosuccinimide (NBS), shown in the second equation below. By using NBS as a brominating agent, allylic brominations are readily achieved in the liquid phase.
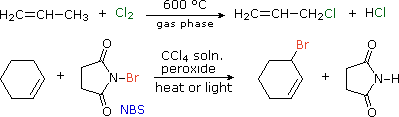
The covalent bond homolyses that define the bond dissociation energies listed above may are described by the general equation:
Since the hydrogen atom is common to all the cases cited here, we can attribute the differences in bond dissociation energies to differences in the stability of the alkyl radicals (R3C·) as the carbon substitution changes. This leads us to the conclusion that:
| alkyl radical stability increases in the order: phenyl < primary (1º) < secondary (2º) < tertiary (3º) < allyl ≈ benzyl. |
Because alkyl radicals are important intermediates in many reactions, this stability relationship will prove to be very useful in future discussions. The enhanced stability of allyl and benzyl radicals may be attributed to resonance stabilization. If you wish to review the principles of resonance Click Here.
Formulas for the allyl and benzyl radicals are shown below. Draw structural formulas for the chief canonical forms contributing to the resonance hybrid in each case.
 |
The poor stability of phenyl radicals, C6H5·, may in turn be attributed to the different hybridization state of the carbon bearing the unpaired electron (sp2 vs. sp3).
