Organic Chemistry Text Book (CHEM 3401 and 3402)
- Home
- Chapter 1: A Review of General ChemistryToggle Dropdown
- 1.1 Introduction to Organic Chemistry
- 1.2 Electrons, Bonds, and Lewis Structures
- 1.3 Identifying Formal Charges
- 1.4 Atomic Orbitals
- 1.5 Valence Bond Theory
- 1.6 Molecular Orbital Theory/Hybridization
- 1.7 VSEPR Theory: Predicting Geometry
- 1.8 Dipole Moments and Molecular Polarity
- 1.9 Intermolecular Forces and Physical Properties
- Problem Set
- Videos for chapter 1
- Chapter 2: Molecular RepresentationsToggle Dropdown
- Chapter 3: Acids and BasesToggle Dropdown
- Chapter 4: Alkanes and CycloalkanesToggle Dropdown
- Chapter 5: StereochemistryToggle Dropdown
- Chapter 6: Chemical Reactivity and MechanismsToggle Dropdown
- Chapter 7: Substitution ReactionsToggle Dropdown
- Chapter 8: Addition Reactions of AlkenesToggle Dropdown
- 8.1 Introduction of Addition Reactions
- 8.1 Nomenclature of Alkenes
- 8.2 Hydrohalogenation of Alkenes
- 8.3 Hydration, Hydroboration, and Oxymercuration of Alkenes
- 8.4 Hydrogenation of Alkenes
- 8.5 Halogenation of Alkenes
- 8.6 Dihydroxylation, Epoxidation, and Ozonolysis of Alkenes
- Problem Set
- Chapter 8 Videos
- Chapter 9: AlkynesToggle Dropdown
- Chapter 10: RadicalsToggle Dropdown
- Chapter 11: SynthesisToggle Dropdown
- Problem Sets Organic Chemistry I (CHEM 3401)
- Chapter 12: Alcohols and PhenolsToggle Dropdown
- 12.1 Alcohol Structure
- 12.2 Solubility
- 12.3 Boiling Point & Melting Point
- 12.4 Nomenclature
- 12.5 Alcohol Acidity
- 12.6 Reactions of Alcohols and Phenols
- 12.6.1 Substitution of the Hydroxyl Hydrogen
- 12.6.2 Nucleophilic Substitution of the Hydroxyl Group
- 12.6.3 Elimination Reactions of Alcohols
- 12.6.4 Oxidation Reactions of Alcohols
- 12.6.5 Reactions of Phenols
- 12.7 Practice Problems
- 12.7.1 Alcohol Nomenclature 1
- 12.7.2 Alcohol Nomenclature 2
- 12.7.3 Alcohol Nomenclature 3
- 12.7.4 Formation of Carbonyl Compounds
- 12.7.5 Functional Relationships of Alcohols
- 12.7.6 Reactions of Alcohols & Phenols
- 12.7.7 Alcohol Reactions
- Chapter 13: Ethers and EpoxidesToggle Dropdown
- Chapter 14: Infrared Spectroscopy and Mass SpectrometryToggle Dropdown
- 14.1 Introduction fo Molecular Spectroscopy
- 14.2 Infrared Spectroscopy
- 14.2.1 Introduction
- 14.2.2 Vibrational Spectroscopy
- 14.2.3 Group Frequencies
- 14.2.4 Table of Characteristic IR Frequencies
- 14.3 Mass Spectrometry
- 14.3.1 The Mass Spectrometer
- 14.3.2 Characteristics of Mass Spectra
- 14.3.3 Isotopes
- 14.3.4 Fragmentation Patterns
- 14.3.5 High Resolution Spectra
- 14.3.6 MS Practice Problems
- 14.3.6a Problem 1
- 14.3.6b Problem 2
- 14.3.6c Problem 3
- 14.3.6d Problem 4
- 14.3.6e Problem 5
- 14.3.6f Problem 6
- 14.3.6g Problem 7
- 14.3.6h Problem 8
- Chapter 15: Nuclear Magnetic Resonance Spectroscopy and UV-Visible SpectroscopyToggle Dropdown
- 15.1 Nuclear Magnetic Resonance Spectroscopy
- 15.1.1 Background
- 15.1.2 Proton NMR Spectroscopy
- 15.1.2a Introduction to Proton NMR Spectroscopy
- 15.1.2b Chemical Shift
- 15.1.2c Signal Strength
- 15.1.2d Hydroxyl Proton Exchange and the Influence of Hydrogen Bonding
- 15.1.2e Pi-Electron Functions
- 15.1.2f Solvent Effects
- 15.1.2g Spin-Spin Interactions
- 15.1.2h Examples
- 15.1.3 Carbon NMR Spectroscopy
- 15.1.4 NMR Practice Problems
- 15.1.4a Problem 1
- 15.1.4b Problem 2
- 15.1.4c Problem 3
- 15.1.4d Problem 4
- 15.1.4e Problem 5
- 15.1.4f Problem 6
- 15.1.4g Problem 7
- 15.1.4h Problem 8
- 15.1.4i Problem 9
- 15.1.4j Problem 10
- 15.1.5 Table of Proton NMR Shifts
- 15.1.6 Table of Carbon NMR Shifts
- 15.2 UV-Visible Spectroscopy
- 15.2.1 Background
- 15.2.2 The Electromagnetic Spectrum
- 15.2.3 UV-Visible Absorption Spectra
- 15.2.4 The Importance of Conjugation
- 15.3 Spectroscopy Practice Problems
- Chapter 16: Conjugated Pi Systems and Pericyclic ReactionsToggle Dropdown
- Chapter 17: Aromatic CompoundsToggle Dropdown
- 17.1 Aromaticity
- 17.1.1 Benzene
- 17.1.2 Fused Ring Compounds
- 17.1.3 Other Aromatic Compounds
- 17.1.4 Antiaromaticity
- 17.1.5 Practice Problems
- 17.1.5a Problem 1
- 17.1.5b Problem 2
- 17.2 Reactions of Substituent Groups
- 17.2.1 Oxidation of Alkyl Side-Chains
- 17.2.2 Bromination of Alkyl Side-Chains
- 17.2.3 Reduction of Nitro Groups
- Chapter 17 Videos
- Chapter 18: Aromatic Substitution ReactionsToggle Dropdown
- 18.1 Electrophilic Aromatic Substitution Reactions
- 18.2 Electrophilic Aromatic Substitution Mechanism
- 18.3 Electrophilic Aromatic Substitution Activation/Deactivation and Orientation
- 18.4 Electrophilic Substitution of Disubstituted Benzene Rings
- 18.5 Practice Problems
- 18.5.1 Problem 1
- 18.5.2 Problem 2
- 18.5.3 Problem 3
- 18.5.4 Problem 4
- 18.5.5 Problem 5
- 18.5.6 Problem 6
- 18.5.7 Problem 7
- Chapter 18 Videos
- Chapter 19: Aldehydes and KetonesToggle Dropdown
- 19.1 Nomenclature
- 19.2 Preparation of Aldehydes and Ketones
- 19.3 Properties of Aldehydes and Ketones
- 19.4 Reactions of Aldehydes and Ketones
- 19.4.1 Addition Reactions
- 19.4.1a Hydration
- 19.4.1b Acetal Formation
- 19.4.1c Imine Formation
- 19.4.1d Cyanohydrin Formation
- 19.4.1e Hydride Reduction
- 19.4.1f Addition of Organometallic Reagents
- 19.4.2 Reduction of Aldehydes and Ketones
- 19.4.2a Wolff-Kishner Reduction
- 19.4.2b Clemmensen Reduction
- 19.4.3 Oxidation of Aldehydes and Ketones
- 19.5 Practice Problems
- 19.5.1 Problem 1
- 19.5.2 Problem 2
- 19.5.3 Problem 3
- 19.5.4 Problem 4
- 19.5.5 Problem 5
- 19.5.6 Problem 6
- 19.5.7 Problem 7
- 19.5.8 Problem 8
- 19.5.9 Problem 9
- 19.5.10 Problem 10
- 19.5.11 Problem 11
- 19.5.12 Problem 12
- Chapter 20: Carboxylic Acids and Their Derivatives
- 20.1 Nomenclature
- 20.2 Physical Properties
- 20.3 Acidity
- 20.4 Preparation of Carboxylic Acids
- 20.5 Reactions of Carboxylic Acids
- 20.5.1 Salt Formation
- 20.5.2 Substitution of the Hydroxyl Hydrogen
- 20.5.3 Substitution of the Hydroxyl Group
- 20.5.4 Reduction
- 20.5.5 Oxidation
- 20.6 Practice Problems-Carboxylic Acids
- 20.6.1 Nomenclature Practice-1
- 20.6.2 Nomenclature Practice-2
- 20.6.3 Acidity
- 20.6.4 Reactions of Carboxylic Acids
- 20.7 Carboxylic Acid Derivatives
- 20.7.1 Related Derivatives
- 20.7.2 Nomenclature
- 20.7.3 Reactions
- 20.7.3a Acyl Substitution
- 20.7.3b Nitrile Hydrolysis
- 20.7.3c Reductions
- 20.7.3d Reactions with Organometallic Reagents
- 20.7.3e Dehydration of Amides
- 20.7.4 Practice Problems-Carboxylic Acid Derivatives
- 20.7.4a Nomenclature Practice-1
- 20.7.4b Nomenclature Practice-2
- 20.7.4c Carbonyl Compounds
- 20.8 Practice Problems
- 20.8.1 Problem 1
- 20.8.2 Problem 2
- 20.8.3 Problem 3
- 20.8.4 Problem 4
- 20.8.5 Problem 5
- 20.8.6 Problem 6
- Chapter 21: Alpha Carbon Chemistry: Enols and EnolatesToggle Dropdown
- 21.1 Reactions at the Alpha Carbon
- 21.2 Alpha Halogenation of Enols and Enolates
- 21.3 Aldol Reaction
- 21.4 Claisen Condensation
- 21.5 Alkylation at the Alpha Position
- 21.5.1 Enolate Alkylation
- 21.5.2 Dicarbonyl Alkylation
- 21.5.3 Decarboxylation Following Alkylation
- 21.5.4 Conjugate Reactions
- 21.5.4a Michael Reaction
- 21.5.4b Robinson Annulation
- 21.5.4c With Hydrides and Organometallics
- 21.6 Practice Problem
- 21.6.1 Problem 1
- Org Chem II - Problem Sets - Collection (CHEM 3402)
- Problem Set
20.7.3a Acyl Substitution
This is probably the single most important reaction of carboxylic acid derivatives. The overall transformation is defined by the following equation, and may be classified either as nucleophilic substitution at an acyl group or as acylation of a nucleophile. For certain nucleophilic reagents the reaction may assume other names as well. If Nuc-H is water the reaction is often called hydrolysis, if Nuc–H is an alcohol the reaction is called alcoholysis, and for ammonia and amines it is called aminolysis.
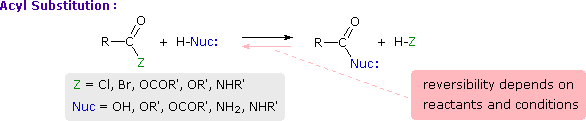
Different carboxylic acid derivatives have very different reactivities, acyl chlorides and bromides being the most reactive and amides the least reactive, as noted in the following qualitatively ordered list. The change in reactivity is dramatic. In homogeneous solvent systems, reaction of acyl chlorides with water occurs rapidly, and does not require heating or catalysts. Amides, on the other hand, react with water only in the presence of strong acid or base catalysts and external heating.
Reactivity: acyl halides > anhydrides >> esters ≈ acids >> amides
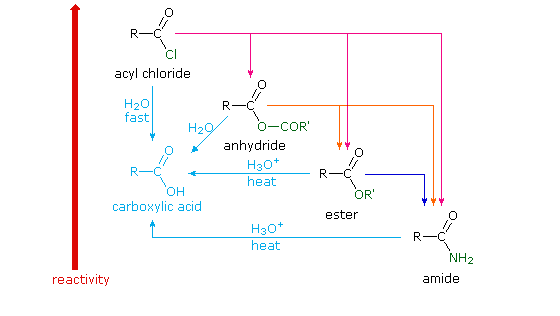
Because of these differences, the conversion of one type of acid derivative into another is generally restricted to those outlined in the following diagram. Methods for converting carboxylic acids into these derivatives were shown in a previous section, but the amide and anhydride preparations were not general and required strong heating. A better and more general anhydride synthesis can be achieved from acyl chlorides, and amides are easily made from any of the more reactive derivatives. Specific examples of these conversions will be displayed by clicking on the product formula. The carboxylic acids themselves are not an essential part of this diagram, although all the derivatives shown can be hydrolyzed to the carboxylic acid state (light blue formulas and reaction arrows). Base catalyzed hydrolysis produces carboxylate salts.
This is probably the single most important reaction of carboxylic acid derivatives. The overall transformation is defined by the following equation, and may be classified either as nucleophilic substitution at an acyl group or as acylation of a nucleophile. For certain nucleophilic reagents the reaction may assume other names as well. If Nuc-H is water the reaction is often called hydrolysis, if Nuc–H is an alcohol the reaction is called alcoholysis, and for ammonia and amines it is called aminolysis.

Different carboxylic acid derivatives have very different reactivities, acyl chlorides and bromides being the most reactive and amides the least reactive, as noted in the following qualitatively ordered list. The change in reactivity is dramatic. In homogeneous solvent systems, reaction of acyl chlorides with water occurs rapidly, and does not require heating or catalysts. Amides, on the other hand, react with water only in the presence of strong acid or base catalysts and external heating.
Reactivity: acyl halides > anhydrides >> esters ≈ acids >> amides
Because of these differences, the conversion of one type of acid derivative into another is generally restricted to those outlined in the following diagram. Methods for converting carboxylic acids into these derivatives were shown in a previous section, but the amide and anhydride preparations were not general and required strong heating. A better and more general anhydride synthesis can be achieved from acyl chlorides, and amides are easily made from any of the more reactive derivatives. Specific examples of these conversions will be displayed by clicking on the product formula. The carboxylic acids themselves are not an essential part of this diagram, although all the derivatives shown can be hydrolyzed to the carboxylic acid state (light blue formulas and reaction arrows). Base catalyzed hydrolysis produces carboxylate salts.

Before proceeding further, it is important to review the general mechanism by means of which all these acyl transfer or acylation reactions take place. Indeed, an alert reader may well be puzzled by the facility of these nucleophilic substitution reactions. After all, it was previously noted that halogens bonded to sp2 or sp hybridized carbon atoms do not usually undergo substitution reactions with nucleophilic reagents. Furthermore, such substitution reactions of alcohols and ethers are rare, except in the presence of strong mineral acids. Clearly, the mechanism by which acylation reactions occur must be different from the SN1 and SN2 procedures described earlier.
In any substitution reaction two things must happen. The bond from the substrate to the leaving group must be broken, and a bond to the replacement group must be formed. The timing of these events may vary with the reacting system. In nucleophilic substitution reactions of alkyl compounds examples of bond-breaking preceding bond-making (the SN1 mechanism), and of bond-breaking and bond-making occurring simultaneously (the SN2 mechanism) were observed. On the other hand, for most cases of electrophilic aromatic substitution bond-making preceded bond-breaking.
As illustrated in the following diagram, acylation reactions generally take place by an addition-elimination process in which a nucleophilic reactant bonds to the electrophilic carbonyl carbon atom to create a tetrahedral intermediate. This tetrahedral intermediate then undergoes an elimination to yield the products. In this two-stage mechanism bond formation occurs before bond cleavage, and the carbonyl carbon atom undergoes a hybridization change from sp2 to sp3 and back again. The facility with which nucleophilic reagents add to a carbonyl group was noted earlier for aldehydes and ketones.
Before proceeding further, it is important to review the general mechanism by means of which all these acyl transfer or acylation reactions take place. Indeed, an alert reader may well be puzzled by the facility of these nucleophilic substitution reactions. After all, it was previously noted that halogens bonded to sp2 or sp hybridized carbon atoms do not usually undergo substitution reactions with nucleophilic reagents. Furthermore, such substitution reactions of alcohols and ethers are rare, except in the presence of strong mineral acids. Clearly, the mechanism by which acylation reactions occur must be different from the SN1 and SN2 procedures described earlier.
In any substitution reaction two things must happen. The bond from the substrate to the leaving group must be broken, and a bond to the replacement group must be formed. The timing of these events may vary with the reacting system. In nucleophilic substitution reactions of alkyl compounds examples of bond-breaking preceding bond-making (the SN1 mechanism), and of bond-breaking and bond-making occurring simultaneously (the SN2 mechanism) were observed. On the other hand, for most cases of electrophilic aromatic substitution bond-making preceded bond-breaking.
As illustrated in the following diagram, acylation reactions generally take place by an addition-elimination process in which a nucleophilic reactant bonds to the electrophilic carbonyl carbon atom to create a tetrahedral intermediate. This tetrahedral intermediate then undergoes an elimination to yield the products. In this two-stage mechanism bond formation occurs before bond cleavage, and the carbonyl carbon atom undergoes a hybridization change from sp2 to sp3 and back again. The facility with which nucleophilic reagents add to a carbonyl group was noted earlier for aldehydes and ketones.
Although amines are among the most reactive nucleophiles, only 1º and 2º-amines give stable amide products. Reaction of 3º-amines with strong acylating reagents may generate acylammonium species reversibly (see below), but these are as reactive as acyl chlorides and will have only a very short existence. This explains why reactions #2 & 3 do not give amide products.
| RCOCl | + | R'3N |  |
RCONR'3(+) Cl(–) (an acylammonium salt) |
Reactions #4 & 5 display the acylating capability of anhydrides. Bear in mind that anhydrides may also be used as reagents in Friedel-Crafts acylation reactions. Esters are less reactive acylating reagents than anhydrides, and the ester exchange reaction (#6) requires a strong acid or base catalyst. The last example demonstrates that nitrogen is generally more nucleophilic than oxygen. Indeed, it is often possible to carry out reactions of amines with acyl chlorides and anhydrides in aqueous sodium hydroxide solution! Not only is the amine more nucleophilic than water, but the acylating reagent is generally not soluble in or miscible with water, reducing the rate of its hydrolysis.
No acylation reactions of amides were shown in these problems. The most important such reaction is hydrolysis, and this normally requires heat and strong acid or base catalysts. One example, illustrating both types of catalysis, is shown here. Mechanisms for catalyzed reactions of this kind were presented earlier.
| R–CO2(–) + CH3NH2 | OH(–) & heat |
|
H(+) & heat |
R–CO2H + CH3NH3(+) |
