Organic Chemistry Text Book (CHEM 3401 and 3402)
- Home
- Chapter 1: A Review of General ChemistryToggle Dropdown
- 1.1 Introduction to Organic Chemistry
- 1.2 Electrons, Bonds, and Lewis Structures
- 1.3 Identifying Formal Charges
- 1.4 Atomic Orbitals
- 1.5 Valence Bond Theory
- 1.6 Molecular Orbital Theory/Hybridization
- 1.7 VSEPR Theory: Predicting Geometry
- 1.8 Dipole Moments and Molecular Polarity
- 1.9 Intermolecular Forces and Physical Properties
- Problem Set
- Videos for chapter 1
- Chapter 2: Molecular RepresentationsToggle Dropdown
- Chapter 3: Acids and BasesToggle Dropdown
- Chapter 4: Alkanes and CycloalkanesToggle Dropdown
- Chapter 5: Stereochemistry
- Chapter 6: Chemical Reactivity and MechanismsToggle Dropdown
- Chapter 7: Substitution ReactionsToggle Dropdown
- Chapter 8: Addition Reactions of AlkenesToggle Dropdown
- 8.1 Introduction of Addition Reactions
- 8.1 Nomenclature of Alkenes
- 8.2 Hydrohalogenation of Alkenes
- 8.3 Hydration, Hydroboration, and Oxymercuration of Alkenes
- 8.4 Hydrogenation of Alkenes
- 8.5 Halogenation of Alkenes
- 8.6 Dihydroxylation, Epoxidation, and Ozonolysis of Alkenes
- Problem Set
- Chapter 8 Videos
- Chapter 9: AlkynesToggle Dropdown
- Chapter 10: RadicalsToggle Dropdown
- Chapter 11: SynthesisToggle Dropdown
- Problem Sets Organic Chemistry I (CHEM 3401)
- Chapter 12: Alcohols and PhenolsToggle Dropdown
- 12.1 Alcohol Structure
- 12.2 Solubility
- 12.3 Boiling Point & Melting Point
- 12.4 Nomenclature
- 12.5 Alcohol Acidity
- 12.6 Reactions of Alcohols and Phenols
- 12.6.1 Substitution of the Hydroxyl Hydrogen
- 12.6.2 Nucleophilic Substitution of the Hydroxyl Group
- 12.6.3 Elimination Reactions of Alcohols
- 12.6.4 Oxidation Reactions of Alcohols
- 12.6.5 Reactions of Phenols
- 12.7 Practice Problems
- 12.7.1 Alcohol Nomenclature 1
- 12.7.2 Alcohol Nomenclature 2
- 12.7.3 Alcohol Nomenclature 3
- 12.7.4 Formation of Carbonyl Compounds
- 12.7.5 Functional Relationships of Alcohols
- 12.7.6 Reactions of Alcohols & Phenols
- 12.7.7 Alcohol Reactions
- Chapter 13: Ethers and EpoxidesToggle Dropdown
- Chapter 14: Infrared Spectroscopy and Mass SpectrometryToggle Dropdown
- 14.1 Introduction fo Molecular Spectroscopy
- 14.2 Infrared Spectroscopy
- 14.2.1 Introduction
- 14.2.2 Vibrational Spectroscopy
- 14.2.3 Group Frequencies
- 14.2.4 Table of Characteristic IR Frequencies
- 14.3 Mass Spectrometry
- 14.3.1 The Mass Spectrometer
- 14.3.2 Characteristics of Mass Spectra
- 14.3.3 Isotopes
- 14.3.4 Fragmentation Patterns
- 14.3.5 High Resolution Spectra
- 14.3.6 MS Practice Problems
- 14.3.6a Problem 1
- 14.3.6b Problem 2
- 14.3.6c Problem 3
- 14.3.6d Problem 4
- 14.3.6e Problem 5
- 14.3.6f Problem 6
- 14.3.6g Problem 7
- 14.3.6h Problem 8
- Chapter 15: Nuclear Magnetic Resonance Spectroscopy and UV-Visible SpectroscopyToggle Dropdown
- 15.1 Nuclear Magnetic Resonance Spectroscopy
- 15.1.1 Background
- 15.1.2 Proton NMR Spectroscopy
- 15.1.2a Introduction to Proton NMR Spectroscopy
- 15.1.2b Chemical Shift
- 15.1.2c Signal Strength
- 15.1.2d Hydroxyl Proton Exchange and the Influence of Hydrogen Bonding
- 15.1.2e Pi-Electron Functions
- 15.1.2f Solvent Effects
- 15.1.2g Spin-Spin Interactions
- 15.1.2h Examples
- 15.1.3 Carbon NMR Spectroscopy
- 15.1.4 NMR Practice Problems
- 15.1.4a Problem 1
- 15.1.4b Problem 2
- 15.1.4c Problem 3
- 15.1.4d Problem 4
- 15.1.4e Problem 5
- 15.1.4f Problem 6
- 15.1.4g Problem 7
- 15.1.4h Problem 8
- 15.1.4i Problem 9
- 15.1.4j Problem 10
- 15.1.5 Table of Proton NMR Shifts
- 15.1.6 Table of Carbon NMR Shifts
- 15.2 UV-Visible Spectroscopy
- 15.2.1 Background
- 15.2.2 The Electromagnetic Spectrum
- 15.2.3 UV-Visible Absorption Spectra
- 15.2.4 The Importance of Conjugation
- 15.3 Spectroscopy Practice Problems
- Chapter 16: Conjugated Pi Systems and Pericyclic ReactionsToggle Dropdown
- Chapter 17: Aromatic CompoundsToggle Dropdown
- 17.1 Aromaticity
- 17.1.1 Benzene
- 17.1.2 Fused Ring Compounds
- 17.1.3 Other Aromatic Compounds
- 17.1.4 Antiaromaticity
- 17.1.5 Practice Problems
- 17.1.5a Problem 1
- 17.1.5b Problem 2
- 17.2 Reactions of Substituent Groups
- 17.2.1 Oxidation of Alkyl Side-Chains
- 17.2.2 Bromination of Alkyl Side-Chains
- 17.2.3 Reduction of Nitro Groups
- Chapter 17 Videos
- Chapter 18: Aromatic Substitution ReactionsToggle Dropdown
- 18.1 Electrophilic Aromatic Substitution Reactions
- 18.2 Electrophilic Aromatic Substitution Mechanism
- 18.3 Electrophilic Aromatic Substitution Activation/Deactivation and Orientation
- 18.4 Electrophilic Substitution of Disubstituted Benzene Rings
- 18.5 Practice Problems
- 18.5.1 Problem 1
- 18.5.2 Problem 2
- 18.5.3 Problem 3
- 18.5.4 Problem 4
- 18.5.5 Problem 5
- 18.5.6 Problem 6
- 18.5.7 Problem 7
- Chapter 18 Videos
- Chapter 19: Aldehydes and KetonesToggle Dropdown
- 19.1 Nomenclature
- 19.2 Preparation of Aldehydes and Ketones
- 19.3 Properties of Aldehydes and Ketones
- 19.4 Reactions of Aldehydes and Ketones
- 19.4.1 Addition Reactions
- 19.4.1a Hydration
- 19.4.1b Acetal Formation
- 19.4.1c Imine Formation
- 19.4.1d Cyanohydrin Formation
- 19.4.1e Hydride Reduction
- 19.4.1f Addition of Organometallic Reagents
- 19.4.2 Reduction of Aldehydes and Ketones
- 19.4.2a Wolff-Kishner Reduction
- 19.4.2b Clemmensen Reduction
- 19.4.3 Oxidation of Aldehydes and Ketones
- 19.5 Practice Problems
- 19.5.1 Problem 1
- 19.5.2 Problem 2
- 19.5.3 Problem 3
- 19.5.4 Problem 4
- 19.5.5 Problem 5
- 19.5.6 Problem 6
- 19.5.7 Problem 7
- 19.5.8 Problem 8
- 19.5.9 Problem 9
- 19.5.10 Problem 10
- 19.5.11 Problem 11
- 19.5.12 Problem 12
- Chapter 20: Carboxylic Acids and Their DerivativesToggle Dropdown
- 20.1 Nomenclature
- 20.2 Physical Properties
- 20.3 Acidity
- 20.4 Preparation of Carboxylic Acids
- 20.5 Reactions of Carboxylic Acids
- 20.5.1 Salt Formation
- 20.5.2 Substitution of the Hydroxyl Hydrogen
- 20.5.3 Substitution of the Hydroxyl Group
- 20.5.4 Reduction
- 20.5.5 Oxidation
- 20.6 Practice Problems-Carboxylic Acids
- 20.6.1 Nomenclature Practice-1
- 20.6.2 Nomenclature Practice-2
- 20.6.3 Acidity
- 20.6.4 Reactions of Carboxylic Acids
- 20.7 Carboxylic Acid Derivatives
- 20.7.1 Related Derivatives
- 20.7.2 Nomenclature
- 20.7.3 Reactions
- 20.7.3a Acyl Substitution
- 20.7.3b Nitrile Hydrolysis
- 20.7.3c Reductions
- 20.7.3d Reactions with Organometallic Reagents
- 20.7.3e Dehydration of Amides
- 20.7.4 Practice Problems-Carboxylic Acid Derivatives
- 20.7.4a Nomenclature Practice-1
- 20.7.4b Nomenclature Practice-2
- 20.7.4c Carbonyl Compounds
- 20.8 Practice Problems
- 20.8.1 Problem 1
- 20.8.2 Problem 2
- 20.8.3 Problem 3
- 20.8.4 Problem 4
- 20.8.5 Problem 5
- 20.8.6 Problem 6
- Chapter 21: Alpha Carbon Chemistry: Enols and EnolatesToggle Dropdown
- 21.1 Reactions at the Alpha Carbon
- 21.2 Alpha Halogenation of Enols and Enolates
- 21.3 Aldol Reaction
- 21.4 Claisen Condensation
- 21.5 Alkylation at the Alpha Position
- 21.5.1 Enolate Alkylation
- 21.5.2 Dicarbonyl Alkylation
- 21.5.3 Decarboxylation Following Alkylation
- 21.5.4 Conjugate Reactions
- 21.5.4a Michael Reaction
- 21.5.4b Robinson Annulation
- 21.5.4c With Hydrides and Organometallics
- 21.6 Practice Problem
- 21.6.1 Problem 1
- Org Chem II - Problem Sets - Collection (CHEM 3402)
- Problem Set
5.2 R and S Configurations
Designating the Configuration of Chiral Centers
Although enantiomers may be identified by their characteristic specific rotations, the assignment of a unique configuration to each has not yet been discussed. We have referred to the mirror-image configurations of enantiomers as "right-handed" and "left-handed", but deciding which is which is not a trivial task. An early procedure assigned a D prefix to enantiomers chemically related to a right-handed reference compound and a L prefix to a similarly related left-handed group of enantiomers. Although this notation is still applied to carbohydrates and amino acids, it required chemical transformations to establish group relationships, and proved to be ambiguous in its general application. A final solution to the vexing problem of configuration assignment was devised by three European chemists: R. S. Cahn, C. K. Ingold and V. Prelog. The resulting nomenclature system is sometimes called the CIP system or the R-S system.
In the CIP system of nomenclature, each chiral center in a molecule is assigned a prefix (R or S), according to whether its configuration is right- or left-handed. No chemical reactions or interrelationship are required for this assignment. The symbol R comes from the Latin rectus for right, and S from the Latin sinister for left. The assignment of these prefixes depends on the application of two rules: The Sequence Rule and The Viewing Rule.
The sequence rule is the same as that used for assigning E-Z prefixes to double bond stereoisomers. Since most of the chiral stereogenic centers we shall encounter are asymmetric carbons, all four different substituents must be ordered in this fashion.
The Sequence Rule for Assignment of Configurations to Chiral CentersAssign sequence priorities to the four substituents by looking at the atoms attached directly to the chiral center. 1. The higher the atomic number of the immediate substituent atom, the higher the priority. |
The Viewing Rule
Once the relative priorities of the four substituents have been determined, the chiral center must be viewed from the side opposite the lowest priority group. If we number the substituent groups from 1 to 4, with 1 being the highest and 4 the lowest in priority sequence, the two enantiomeric configurations are shown in the following diagram along with a viewers eye on the side opposite substituent #4.

Remembering the geometric implication of wedge and hatched bonds, an observer (the eye) notes whether a curved arrow drawn from the # 1 position to the # 2 location and then to the # 3 position turns in a clockwise or counter-clockwise manner. If the turn is clockwise, as in the example on the right, the configuration is classified R. If it is counter-clockwise, as in the left illustration, the configuration is S. Another way of remembering the viewing rule, is to think of the asymmetric carbon as a steering wheel. The bond to the lowest priority group (# 4) is the steering column, and the other bonds are spokes on the wheel. If the wheel is turned from group # 1 toward group # 2, which in turn moves toward group # 3, this would either negotiate a right turn (R) or a left turn (S). This model is illustrated below for a right-handed turn, and the corresponding (R)-configurations of lactic acid and carvone are shown to its right. The stereogenic carbon atom is colored magenta in each case, and the sequence priorities are shown as light blue numbers. Note that if any two substituent groups on a stereogenic carbon are exchanged or switched, the configuration changes to its mirror image.
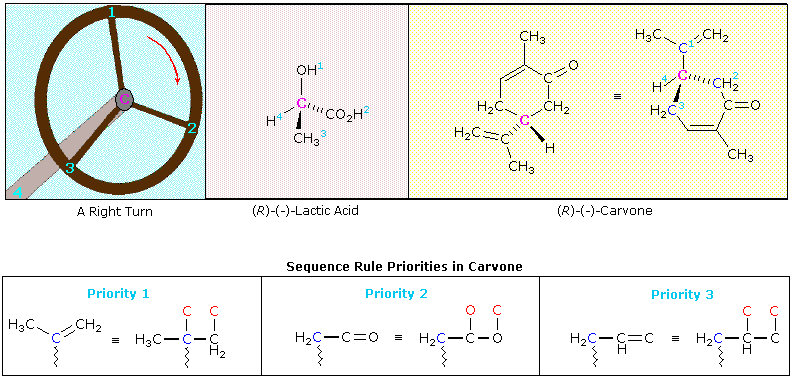
The sequence order of the substituent groups in lactic acid should be obvious, but the carvone example requires careful analysis. The hydrogen is clearly the lowest priority substituent, but the other three groups are all attached to the stereogenic carbon by bonds to carbon atoms (colored blue here). Two of the immediate substituent species are methylene groups (CH2), and the third is a doubly-bonded carbon. Rule # 3 of the sequence rules allows us to order these substituents. The carbon-carbon double bond is broken so as to give imaginary single-bonded carbon atoms (the phantom atoms are colored red in the equivalent structure). In this form the double bond assumes the priority of a 3º-alkyl group, which is greater than that of a methylene group. To establish the sequence priority of the two methylene substituents (both are part of the ring), we must move away from the chiral center until a point of difference is located. This occurs at the next carbon, which on one side is part of a carbonyl double bond (C=O), and on the other, part of a carbon-carbon double bond. Rule # 3 is again used to evaluate the two cases. The carbonyl group places two oxygens (one phantom) on the adjacent carbon atom, so this methylene side is ranked ahead of the other.
An interesting feature of the two examples shown here is that the R-configuration in both cases is levorotatory (-) in its optical activity. The mirror-image S-configurations are, of course, dextrorotatory (+). It is important to remember that there is no simple or obvious relationship between the R or S designation of a molecular configuration and the experimentally measured specific rotation of the compound it represents. In order to determine the true or "absolute" configuration of an enantiomer, as in the cases of lactic acid and carvone reported here, it is necessary either to relate the compound to a known reference structure, or to conduct a rather complex X-ray analysis on a single crystal of the sample.
The configurations of lactic acid and carvone enantiomers may be examined as interactive models by
.
| The module on the right provides examples of chiral and achiral molecules for analysis. These are displayed as three-dimensional structures which may be moved about and examined from various points of view. By using this resource the reader's understanding of configurational notation may be tested. This visualization makes use of the Jmol applet. With some browsers it may be necessary to click a button twice for action.
|
|
|||||
| A response to your selection will appear here. A sequence assignment will be shown above. |
Configurational drawings of chiral molecules sometimes display structures in a way that does not permit an easy application of the viewing rule. In the example of carvone, shown above, the initial formula directed the lowest priority substituent (H) toward the viewer, requiring either a reorientation display or a very good sense of three-dimensional structure on the part of the reader. The Fischer projection formulas, described later, are another example of displays that challenge even experienced students. A useful mnemonic, suggested by Professor Michael Rathke, is illustrated below. Here a stereogenic tetrahedral carbon has four different substituents, designated 1, 2, 3 & 4. If we assume that these numbers represent the sequence priority of these substituents (1 > 2 > 3 > 4), then the R and S configurations are defined.
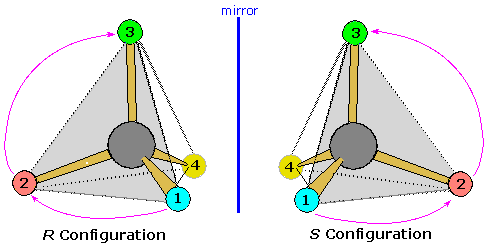
The viewing rule states that when the lowest priority substituent (4) is oriented behind the triangular face defined by the three higher priority substituents (shaded light gray here), a clockwise sequential arrangement of these substituents (1, 2 & 3) is defined as R, and a counter-clockwise sequence as S.
Now a tetrahedral structure may be viewed from any of the four triangular faces, and the symmetry of the system is such that a correct R/S assignment is made if the remote out-of plane group has an even number sequence priority (2 or 4), whereas the wrong assignment results when the out-of plane group has an odd priority (1 or 3). Once one recognizes this relationship, the viewing options are increased and a configurational assignment is more easily achieved. For an example, click on the diagram to see the 1:3:4-face, shaded light gray. oriented in front of substituent 2. Note that the R/S assignment is unchanged.
Two or More Chiral Centers |
|---|
Compounds Having Two or More Chiral Centers
The Chinese shrub Ma Huang (Ephedra vulgaris) contains two physiologically active compounds ephedrine and pseudoephedrine. Both compounds are stereoisomers of 2-methylamino-1-phenyl-1-propanol, and both are optically active, one being levorotatory and the other dextrorotatory. Since the properties of these compounds (see below) are significantly different, they cannot be enantiomers. How, then, are we to classify these isomers and others like them?
| Ephedrine from Ma Huang: | m.p. 35 - 40 º C, [α]D = –41º, moderate water solubility | [this isomer may be referred to as (–)-ephedrine] |
| Pseudoephedrine from Ma Huang: | m.p. 119 º C, [α]D = +52º, relatively insoluble in water | [this isomer may be referred to as (+)-pseudoephedrine] |
Since these two compounds are optically active, each must have an enantiomer. Although these missing stereoisomers were not present in the natural source, they have been prepared synthetically and have the expected identical physical properties and opposite-sign specific rotations with those listed above. The structural formula of 2-methylamino-1-phenylpropanol has two stereogenic carbons (#1 & #2). Each may assume an R or S configuration, so there are four stereoisomeric combinations possible. These are shown in the following illustration, together with the assignments that have been made on the basis of chemical interconversions.
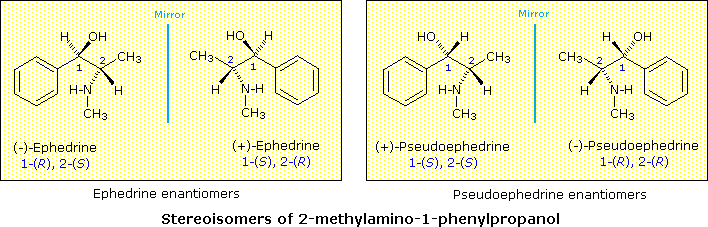
As a general rule, a structure having n chiral centers will have 2n possible combinations of these centers. Depending on the overall symmetry of the molecular structure, some of these combinations may be identical, but in the absence of such identity, we would expect to find 2n stereoisomers. Some of these stereoisomers will have enantiomeric relationships, but enantiomers come in pairs, and non-enantiomeric stereoisomers will therefore be common. We refer to such stereoisomers as diastereomers. In the example above, either of the ephedrine enantiomers has a diastereomeric relationship with either of the pseudoephedrine enantiomers.
