Organic Chemistry Text Book (CHEM 3401 and 3402)
- Home
- Chapter 1: A Review of General ChemistryToggle Dropdown
- 1.1 Introduction to Organic Chemistry
- 1.2 Electrons, Bonds, and Lewis Structures
- 1.3 Identifying Formal Charges
- 1.4 Atomic Orbitals
- 1.5 Valence Bond Theory
- 1.6 Molecular Orbital Theory/Hybridization
- 1.7 VSEPR Theory: Predicting Geometry
- 1.8 Dipole Moments and Molecular Polarity
- 1.9 Intermolecular Forces and Physical Properties
- Problem Set
- Videos for chapter 1
- Chapter 2: Molecular RepresentationsToggle Dropdown
- Chapter 3: Acids and BasesToggle Dropdown
- Chapter 4: Alkanes and CycloalkanesToggle Dropdown
- Chapter 5: StereochemistryToggle Dropdown
- Chapter 6: Chemical Reactivity and MechanismsToggle Dropdown
- Chapter 7: Substitution ReactionsToggle Dropdown
- Chapter 8: Addition Reactions of AlkenesToggle Dropdown
- 8.1 Introduction of Addition Reactions
- 8.1 Nomenclature of Alkenes
- 8.2 Hydrohalogenation of Alkenes
- 8.3 Hydration, Hydroboration, and Oxymercuration of Alkenes
- 8.4 Hydrogenation of Alkenes
- 8.5 Halogenation of Alkenes
- 8.6 Dihydroxylation, Epoxidation, and Ozonolysis of Alkenes
- Problem Set
- Chapter 8 Videos
- Chapter 9: AlkynesToggle Dropdown
- Chapter 10: Radicals
- Chapter 11: SynthesisToggle Dropdown
- Problem Sets Organic Chemistry I (CHEM 3401)
- Chapter 12: Alcohols and PhenolsToggle Dropdown
- 12.1 Alcohol Structure
- 12.2 Solubility
- 12.3 Boiling Point & Melting Point
- 12.4 Nomenclature
- 12.5 Alcohol Acidity
- 12.6 Reactions of Alcohols and Phenols
- 12.6.1 Substitution of the Hydroxyl Hydrogen
- 12.6.2 Nucleophilic Substitution of the Hydroxyl Group
- 12.6.3 Elimination Reactions of Alcohols
- 12.6.4 Oxidation Reactions of Alcohols
- 12.6.5 Reactions of Phenols
- 12.7 Practice Problems
- 12.7.1 Alcohol Nomenclature 1
- 12.7.2 Alcohol Nomenclature 2
- 12.7.3 Alcohol Nomenclature 3
- 12.7.4 Formation of Carbonyl Compounds
- 12.7.5 Functional Relationships of Alcohols
- 12.7.6 Reactions of Alcohols & Phenols
- 12.7.7 Alcohol Reactions
- Chapter 13: Ethers and EpoxidesToggle Dropdown
- Chapter 14: Infrared Spectroscopy and Mass SpectrometryToggle Dropdown
- 14.1 Introduction fo Molecular Spectroscopy
- 14.2 Infrared Spectroscopy
- 14.2.1 Introduction
- 14.2.2 Vibrational Spectroscopy
- 14.2.3 Group Frequencies
- 14.2.4 Table of Characteristic IR Frequencies
- 14.3 Mass Spectrometry
- 14.3.1 The Mass Spectrometer
- 14.3.2 Characteristics of Mass Spectra
- 14.3.3 Isotopes
- 14.3.4 Fragmentation Patterns
- 14.3.5 High Resolution Spectra
- 14.3.6 MS Practice Problems
- 14.3.6a Problem 1
- 14.3.6b Problem 2
- 14.3.6c Problem 3
- 14.3.6d Problem 4
- 14.3.6e Problem 5
- 14.3.6f Problem 6
- 14.3.6g Problem 7
- 14.3.6h Problem 8
- Chapter 15: Nuclear Magnetic Resonance Spectroscopy and UV-Visible SpectroscopyToggle Dropdown
- 15.1 Nuclear Magnetic Resonance Spectroscopy
- 15.1.1 Background
- 15.1.2 Proton NMR Spectroscopy
- 15.1.2a Introduction to Proton NMR Spectroscopy
- 15.1.2b Chemical Shift
- 15.1.2c Signal Strength
- 15.1.2d Hydroxyl Proton Exchange and the Influence of Hydrogen Bonding
- 15.1.2e Pi-Electron Functions
- 15.1.2f Solvent Effects
- 15.1.2g Spin-Spin Interactions
- 15.1.2h Examples
- 15.1.3 Carbon NMR Spectroscopy
- 15.1.4 NMR Practice Problems
- 15.1.4a Problem 1
- 15.1.4b Problem 2
- 15.1.4c Problem 3
- 15.1.4d Problem 4
- 15.1.4e Problem 5
- 15.1.4f Problem 6
- 15.1.4g Problem 7
- 15.1.4h Problem 8
- 15.1.4i Problem 9
- 15.1.4j Problem 10
- 15.1.5 Table of Proton NMR Shifts
- 15.1.6 Table of Carbon NMR Shifts
- 15.2 UV-Visible Spectroscopy
- 15.2.1 Background
- 15.2.2 The Electromagnetic Spectrum
- 15.2.3 UV-Visible Absorption Spectra
- 15.2.4 The Importance of Conjugation
- 15.3 Spectroscopy Practice Problems
- Chapter 16: Conjugated Pi Systems and Pericyclic ReactionsToggle Dropdown
- Chapter 17: Aromatic CompoundsToggle Dropdown
- 17.1 Aromaticity
- 17.1.1 Benzene
- 17.1.2 Fused Ring Compounds
- 17.1.3 Other Aromatic Compounds
- 17.1.4 Antiaromaticity
- 17.1.5 Practice Problems
- 17.1.5a Problem 1
- 17.1.5b Problem 2
- 17.2 Reactions of Substituent Groups
- 17.2.1 Oxidation of Alkyl Side-Chains
- 17.2.2 Bromination of Alkyl Side-Chains
- 17.2.3 Reduction of Nitro Groups
- Chapter 17 Videos
- Chapter 18: Aromatic Substitution ReactionsToggle Dropdown
- 18.1 Electrophilic Aromatic Substitution Reactions
- 18.2 Electrophilic Aromatic Substitution Mechanism
- 18.3 Electrophilic Aromatic Substitution Activation/Deactivation and Orientation
- 18.4 Electrophilic Substitution of Disubstituted Benzene Rings
- 18.5 Practice Problems
- 18.5.1 Problem 1
- 18.5.2 Problem 2
- 18.5.3 Problem 3
- 18.5.4 Problem 4
- 18.5.5 Problem 5
- 18.5.6 Problem 6
- 18.5.7 Problem 7
- Chapter 18 Videos
- Chapter 19: Aldehydes and KetonesToggle Dropdown
- 19.1 Nomenclature
- 19.2 Preparation of Aldehydes and Ketones
- 19.3 Properties of Aldehydes and Ketones
- 19.4 Reactions of Aldehydes and Ketones
- 19.4.1 Addition Reactions
- 19.4.1a Hydration
- 19.4.1b Acetal Formation
- 19.4.1c Imine Formation
- 19.4.1d Cyanohydrin Formation
- 19.4.1e Hydride Reduction
- 19.4.1f Addition of Organometallic Reagents
- 19.4.2 Reduction of Aldehydes and Ketones
- 19.4.2a Wolff-Kishner Reduction
- 19.4.2b Clemmensen Reduction
- 19.4.3 Oxidation of Aldehydes and Ketones
- 19.5 Practice Problems
- 19.5.1 Problem 1
- 19.5.2 Problem 2
- 19.5.3 Problem 3
- 19.5.4 Problem 4
- 19.5.5 Problem 5
- 19.5.6 Problem 6
- 19.5.7 Problem 7
- 19.5.8 Problem 8
- 19.5.9 Problem 9
- 19.5.10 Problem 10
- 19.5.11 Problem 11
- 19.5.12 Problem 12
- Chapter 20: Carboxylic Acids and Their DerivativesToggle Dropdown
- 20.1 Nomenclature
- 20.2 Physical Properties
- 20.3 Acidity
- 20.4 Preparation of Carboxylic Acids
- 20.5 Reactions of Carboxylic Acids
- 20.5.1 Salt Formation
- 20.5.2 Substitution of the Hydroxyl Hydrogen
- 20.5.3 Substitution of the Hydroxyl Group
- 20.5.4 Reduction
- 20.5.5 Oxidation
- 20.6 Practice Problems-Carboxylic Acids
- 20.6.1 Nomenclature Practice-1
- 20.6.2 Nomenclature Practice-2
- 20.6.3 Acidity
- 20.6.4 Reactions of Carboxylic Acids
- 20.7 Carboxylic Acid Derivatives
- 20.7.1 Related Derivatives
- 20.7.2 Nomenclature
- 20.7.3 Reactions
- 20.7.3a Acyl Substitution
- 20.7.3b Nitrile Hydrolysis
- 20.7.3c Reductions
- 20.7.3d Reactions with Organometallic Reagents
- 20.7.3e Dehydration of Amides
- 20.7.4 Practice Problems-Carboxylic Acid Derivatives
- 20.7.4a Nomenclature Practice-1
- 20.7.4b Nomenclature Practice-2
- 20.7.4c Carbonyl Compounds
- 20.8 Practice Problems
- 20.8.1 Problem 1
- 20.8.2 Problem 2
- 20.8.3 Problem 3
- 20.8.4 Problem 4
- 20.8.5 Problem 5
- 20.8.6 Problem 6
- Chapter 21: Alpha Carbon Chemistry: Enols and EnolatesToggle Dropdown
- 21.1 Reactions at the Alpha Carbon
- 21.2 Alpha Halogenation of Enols and Enolates
- 21.3 Aldol Reaction
- 21.4 Claisen Condensation
- 21.5 Alkylation at the Alpha Position
- 21.5.1 Enolate Alkylation
- 21.5.2 Dicarbonyl Alkylation
- 21.5.3 Decarboxylation Following Alkylation
- 21.5.4 Conjugate Reactions
- 21.5.4a Michael Reaction
- 21.5.4b Robinson Annulation
- 21.5.4c With Hydrides and Organometallics
- 21.6 Practice Problem
- 21.6.1 Problem 1
- Org Chem II - Problem Sets - Collection (CHEM 3402)
- Problem Set
10.1 Introduction of Radicals
Organic Radicals
1. Background & Introduction
A radical is an atomic or molecular species having an unpaired, or odd, electron. Some radicals, such as nitric oxide (NO), are relatively stable, but most are so reactive that their isolation and long-term study is not possible under normal laboratory conditions. The electrons in most stable organic compounds are paired in atomic or molecular orbitals, so the total electron count is an even number. Molecular oxygen (O2) is a rare example of a stable biradical (two unpaired electrons having the same spin), with an even number of electrons.
Early chemists used the term "radical" for nomenclature purposes, much as we now use the term "group". Many doubted that such open-valenced species could exist, although there was circumstantial evidence for their participation in gas phase reactions. Credit for the first isolation and characterization of a "free radical" goes to Moses Gomberg, a young instructor at the University of Michigan. In 1900 Gomberg attempted a synthesis of hexaphenylethane by reacting triphenylmethyl chloride with finely divided metals such as silver and zinc. When air was excluded from the reaction, he obtained a yellow solution, the color of which darkened reversibly on heating and cooling. This solution yielded a colorless, crystalline C38H30 hydrocarbon which Gomberg assumed to be hexaphenylethane.
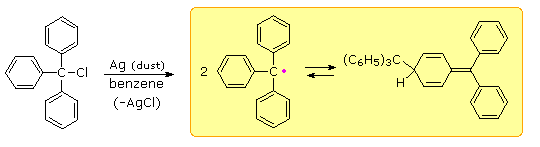
If the yellow solution was exposed to air (or oxygen) a C38H30O2 peroxide was obtained, and identified by reduction to the known alcohol, triphenylmethanol. In a similar fashion the yellow solution reacted with iodine to produce triphenylmethyl iodide. These reactions will be displayed by clicking on the diagram above. Gomberg concluded that the colored solutions contained reactive triphenylmethyl free radicals, formed by thermal dissociation of their dimer (Keq = 2 • 10–4 at 25º C). The exceptional stability of this carbon radical is attributed to odd electron delocalization into the three phenyl rings. Discrete Kekule formulas demonstrate that this benzyl-like delocalization places the electron on ortho and para carbons, but not on meta carbons. Clicking on the diagram a third time will display this delocalization in a general way.
The resonance structures drawn here may give the impression that the triphenylmethyl radical is planar (flat). Actually the phenyl groups are turned by about 35º, producing a shape similar to a three bladed propellor. Despite this twist, the p-pi orbital overlap is still over 80%, so the electron delocalization is not seriously diminished. To see a model of this unusual radical
.
More than fifty years later, the reactive dimer of triphenylmethyl radical was shown to be the para-coupled compound drawn above and not hexaphenylethane. The steric crowding of phenyl groups in the simple ethane dimer is apparently so severe that bonding between two 3º-carbon atoms is prohibited. Since the electron delocalization noted above places radical character at the para carbons of the phenyl groups, bonding to this relatively unhindered location is preferred, although at the cost of one benzene ring's aromaticity. If the para-locations are themselves hindered by large meta substituents, then an unstable hexaarylethane may actually be formed.
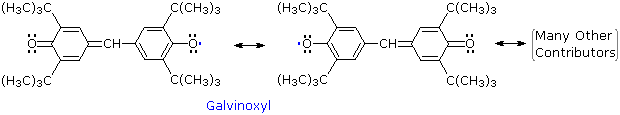
Other relatively stable radicals, such as galvinoxyl have been prepared and studied. These species usually owe their stability to a combination of odd electron delocalization and steric hindrance to dimerization, as the ortho tert-butyl groups in galvinoxyl demonstrate. The term "free radical" is now loosely applied to all radical intermediates, stabilized or not.
2. Detection and Observation of Radicals
Only triphenymethyl and a few other stabilized radicals may be generated in concentrations suitable for examination by traditional laboratory methods. Evidence for the transient existence of more reactive radical species in chemical reactions usually requires special techniques, including low-temperature isolation in solids and high speed spectroscopic probes. However, an interesting chemical detection of the methyl radical was carried out by the Austrian chemist Fritz Paneth not long after Gomberg's preparation of triphenylmethyl radical. The Paneth experiment involved gas phase thermal decomposition of tetramethyllead to methyl radicals and lead atoms in a glass tube. The initial flow of the lead compound through the tube is shown in the following illustration, and the consequences of applying strong heat to the tube will be displayed by repetitive clicking on the diagram. The text box beneath the diagram provides commentary.
.
 |
|---|
Electron Paramagnetic Resonance
The same unpaired or odd electron that renders most radical intermediates unstable and highly reactive may be induced to leave a characteristic "calling card" by a magnetic resonance phenomenon called "electron spin resonance" (esr) or "electron paramagnetic resonance" (epr). Just as a proton (spin = 1/2) will occupy one of two energy states in a strong external magnetic field, giving rise to nmr spectroscopy; an electron (spin = 1/2) may also assume two energy states in an external field. Because the magnetic moment of an electron is roughly a thousand times larger than that of a proton, the energy difference between the spin states falls in the microwave region of the spectrum (assuming a moderate magnetic field strength). The lifetime of electron spin states is much shorter than nuclear spin states, so esr absorptions are much broader than nmr signals. One way of improving the signal to noise ratio in esr spectra is to display them as first derivatives rather than absorptions. These displays are illustrated on the left below. In practice, esr spectra may be quite complex, as shown by the derivative spectrum of triphenylmethyl radical on the right. This complexity is the result of hyperfine splitting of the resonance signal by protons and other nuclear spins, an interaction similar to spin-spin splitting in nmr spectroscopy. For example, the esr signal from methyl radicals, generated by x-radiation of solid methyl iodide at -200º C, is a 1:3:3:1 quartet (predicted by the n + 1 rule). The magnitude of signal splitting is much larger than nmr coupling constants (MHz rather than Hz), and is usually reported in units of gauss. The complexity of the triphenylmethyl spectrum is due to three different hyperfine splittings: 3 para hydrogens, 6 ortho hydrogens & 6 meta hydrogens. Ideally this should produce 196 lines, but imperfect resolution reduces the number observed.
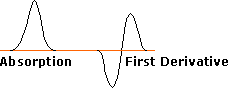
ESR Signal Types |
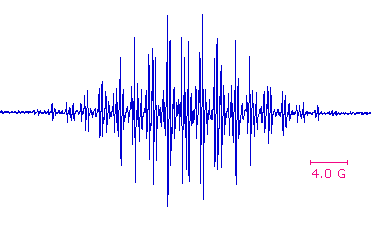 Triphenylmethyl ESR Spectrum |
|---|
3. Methods of Generating Free Radicals
The homolytic cleavage of covalent bonds produces radicals, and since this is an endothermic process, it requires the introduction of energy from the surroundings. Heat serves this purpose by collisional interconversion of kinetic energy into vibrational energy, and the temperature required for bond homolysis will be proportional to the bond dissociation energy. Absorption of light may also lead to radical species by intra- or intermolecular conversion of the increased electronic energy into vibrational energy. As expected, weaker covalent bonds dissociate into radicals more readily than stronger covalent bonds. The following table lists standard bond energies (D) for the C–C, C–O and C–H bonds commonly found in organic compounds, together with bond energies for some weaker bonds that have been found useful for generating radicals. Approximate homolysis temperatures at which half the bonds are cleaved in one hour are also given.
Some Standard Bond Energies and Approximate Homolysis Temperatures
| Bond | D kcal/mole |
T ºC | Bond | D kcal/mole |
T ºC | Bond | D kcal/mole |
T ºC | ||
| C–C | 85 | 670 | O–O | 34 | 160 | O–Cl | 49 | 280 | ||
| C–H | 99 | 850 | N–N | 39 | 230 | C–I | 51 | 350 | ||
| C–O | 84 | 680 | S–S | 55 | 440 | C–Br | 67 | 480 |
A. Thermal Cracking
At temperatures greater than 500º C, and in the absence of oxygen, mixtures of high molecular weight alkanes break down into smaller alkane and alkene fragments. This cracking process is important in the refining of crude petroleum because of the demand for lower boiling gasoline fractions. Free radicals, produced by homolysis of C–C bonds, are known to be intermediates in these transformations. Studies of model alkanes have shown that highly substituted C–C bonds undergo homolysis more readily than do unbranched alkanes. In practice, catalysts are used to lower effective cracking temperatures.
B. Homolysis of Peroxides and Azo Compounds
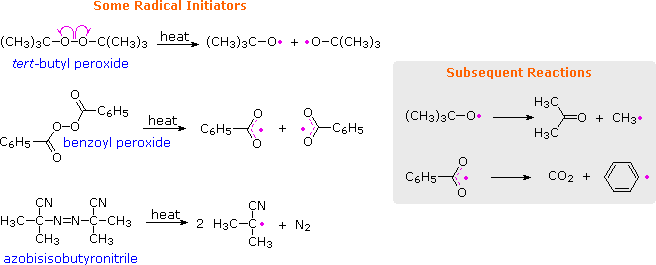
In contrast to stronger C–C and C–H bonds, the very weak O–O bonds of peroxides are cleaved at relatively low temperatures ( 80 to 150 ºC ), as shown in the following equations. The resulting oxy radicals may then initiate other reactions, or may decompose to carbon radicals, as noted in the shaded box. The most commonly used peroxide initiators are depicted in the first two equations.
Organic azo compounds (R–N=N–R) are also heat sensitive, decomposing to alkyl radicals and nitrogen. Azobisisobutyronitrile (AIBN) is the most widely used radical initiator of this kind, decomposing slightly faster than benzoyl peroxide at 70 to 80 ºC. The thermodynamic stability of nitrogen provides an overall driving force for this decomposition, but its favorable rate undoubtedly reflects weaker than normal C-N bonds.
C. Photolytic Bond Homolysis
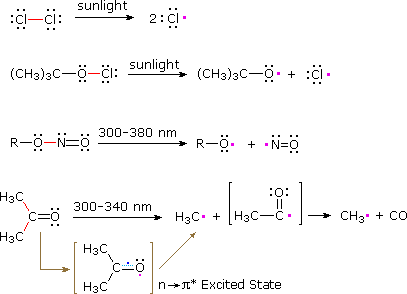
Compounds having absorption bands in the visible or near ultraviolet spectrum may be electronically excited to such a degree that weak covalent bonds undergo homolysis. Examples include the halogens Cl2, Br2 & I2 (bond dissociation energies are 58, 46 & 36 kcal/mole respectively), alkyl hypochlorites, nitrite esters and ketones. Equations illustrating these radical producing reactions are displayed below. The covalent bonds that undergo homolysis are colored red, and the unpaired electrons in the resulting radicals are colored pink. Ketones undergo n to π* electronic excitation near 300 nm. The resulting excited state is a diradical in which one of the odd electrons is localized on the oxygen atom. Cleavage of an alkyl group may then take place.
D. Electron Transfer
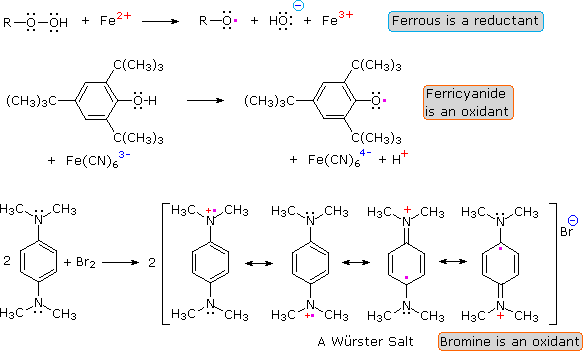
The action of inorganic oxidizing and reducing agents on organic compounds may involve electron transfers that produce radical or radical ionic species. Ferrous ion, for example, catalyzes the decomposition of hydrogen peroxide ( Fenton's reagent ) and organic peroxides. In some cases the radical intermediates formed in this manner are sufficiently stable to be studied in the absence of oxygen. The phenoxy radical formed in the second equation below is one such species, Würster's salt ( third equation ) is another.
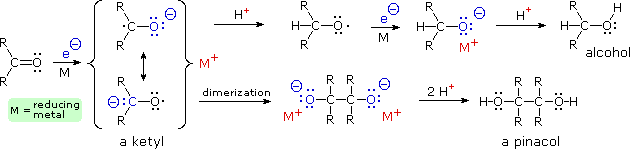
The alkali metals lithium, sodium and potassium reduce the carbonyl group of ketones to a deep blue radical anion called a "ketyl", shown in the following illustration. Subsequent chemical reactions of these useful intermediates are discussed elsewhere. A similar reduction of benzene and its derivatives also proceeds by way of radical anion intermediates.
E. Hydrogen and Halogen Atom Abstraction
If free radical reactions are to be useful to organic chemists, methods for transferring the reactivity of the simple radicals generated by the previously described homolysis reactions to specific sites in substrate molecules must be devised. The most direct way of doing this is by an atom abstraction, as shown here.
R–H + X• –––> R• + H–X
Indeed, when X is Cl or Br, this is a key step in the alkane halogenation chain reaction. Hydrogen abstraction reactions of this kind are sensitive to the nature of both the attacking radical ( X•) and the R–H bond. This is illustrated by the relative rates of hydrogen abstraction given in the following table. Each horizontal row of data is normalized to 1º C–H (1.0), but there are also large differences between rows. Thus the rate of reaction of 1º C–H with Cl• is a thousand times faster than with Br•. However, the less reactive bromine atom shows much greater selectivity in discriminating between 1º, 2º and 3º C–H groups.
Certain C–H bonds are so susceptible to radical attack that they react with atmospheric oxygen (a diradical) to form peroxides. Typical groups that exhibit this trait are 3º-alkyl, 2º & 3º-benzyl and alkoxy groups in ethers.
R–H + O2 –––> R• + •O2H –––> R–O–O–H
The exceptional facility with which S–H and Sn–H react with alkyl radicals makes thiophenol and trialkyltin hydrides excellent radical quenching agents, when present in excess. At equimolar or lower concentration they function well as radical transfer agents..
Relative Reactivities (per hydrogen) of Hydrogen Atom Donors with Selected Radicals
|
Hydrogen Donor |
C2H6 |
RCH2R |
R3CH |
C6H5CH3 |
CH2=CH-CH2R |
RCOCH3 |
C6H5SH |
(C4H9)3SnH |
|---|---|---|---|---|---|---|---|---|
|
Attacking Radical |
||||||||
|
CH3• |
1.0 | 10 | 100 | 85 | 25 | 170 | 4•107 | 5•105 |
|
(CH3)3CO• |
1.0 | 6 | 15 | 10 | 0.5 | 3.5 | -- | 104 |
|
Cl• |
1.0 | 5 | 6 | 2 | -- | -- | -- | -- |
|
Br• |
1.0 | 220 | 2•104 | 5•104 | -- | -- | -- | -- |
Carbon halogen bonds, especially C–Br and C–I, are weaker than C–H bonds and react with alkyl and stannyl radicals to generate new alkyl radicals. This reaction has been put to practical use in a mild procedure for reducing alkyl halides to alkanes. The chain reaction sequence that accomplishes this reduction is shown here. By clicking on the diagram, three examples of this dehalogenation reaction will be displayed.
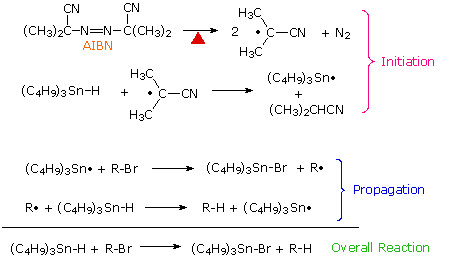
An important modification of this reduction is shown in the third example above. The use of equimolar amounts of tributyltin hydride in reactions presents certain problems, including the toxicity presented by organostannanes, difficulty in separating nonpolar stannanes, such as halides, bis(tributyltin) and bis(tributyltin) oxide from desired products, and formation of tin oxides by reaction with moisture. To reduce these difficulties, a catalytic amount of the stannanes may be used together with enough NaBH4 (or an equivalent reagent) to convert the tributyltin halides to the hydride. Indeed, the reduction is so facile that traces of peroxides in the reactants often initiate reaction without added AIBN.
4. The Configuration of Free Radicals
The configurational preferences of different reactive intermediates were noted in an earlier section. Since the difference in energy between a planar radical and a rapidly inverting pyramidal radical is small, radicals generated at chiral centers generally lead to racemic products. However, unlike carbocation intermediates, which prefer to be planar, radicals tolerate being restricted to a pyramidal configuration. The following illustration shows the decomposition of a bicyclic bridgehead acyl peroxide. Initial formation of a carboxyl radical is followed by loss of carbon dioxide to give a pyramidal bridgehead radical. This radical abstracts a chlorine atom from the solvent, yielding the bridgehead chloride as the major product. Although this is a 3º-alkyl halide, it does not undergo SN1 solvolysis reactions because of the strain imposed on the carbocation intermediate by its pyramidal confinement.
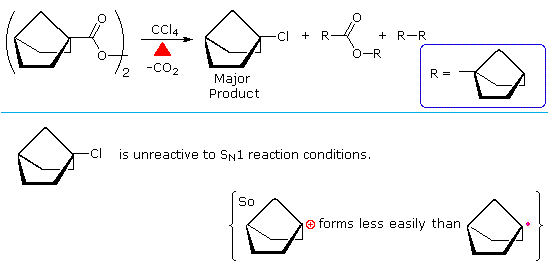
The concurrent formation of ester and dimeric cycloalkane products from acyl peroxides is common, and reflects a cage effect in homolysis reactions. When a pair of radicals is formed by homolysis, they are briefly held in proximity by the surrounding solvent molecules (the cage). Rapid decomposition to other radicals may occur, but until one or both of these radicals escape the solvent cage a significant degree of coupling (recombination) may occur. A general description of the cage effect will be displayed above by clicking on the diagram.
Cage recombination of radicals may be sufficiently rapid to preserve the configuration of the generating species. An example will be shown above by clicking on the diagram a second time. Ester formation is clearly a cage product, whereas 2-chloro-1-phenylpropane comes largely from radicals that have escaped the cage and lost configurational identity. The chiral centers in these compounds are marked by asterisks.
