Organic Chemistry Text Book (CHEM 3401 and 3402)
- Home
-
Chapter 1: A Review of General Chemistry
Toggle Dropdown
- 1.1 Introduction to Organic Chemistry
- 1.2 Electrons, Bonds, and Lewis Structures
- 1.3 Identifying Formal Charges
- 1.4 Atomic Orbitals
- 1.5 Valence Bond Theory
- 1.6 Molecular Orbital Theory/Hybridization
- 1.7 VSEPR Theory: Predicting Geometry
- 1.8 Dipole Moments and Molecular Polarity
- 1.9 Intermolecular Forces and Physical Properties
- Problem Set
- Videos for chapter 1
- Chapter 2: Molecular Representations Toggle Dropdown
- Chapter 3: Acids and Bases Toggle Dropdown
- Chapter 4: Alkanes and Cycloalkanes Toggle Dropdown
- Chapter 5: Stereochemistry Toggle Dropdown
- Chapter 6: Chemical Reactivity and Mechanisms Toggle Dropdown
- Chapter 7: Substitution Reactions Toggle Dropdown
-
Chapter 8: Addition Reactions of Alkenes
Toggle Dropdown
- 8.1 Introduction of Addition Reactions
- 8.1 Nomenclature of Alkenes
- 8.2 Hydrohalogenation of Alkenes
- 8.3 Hydration, Hydroboration, and Oxymercuration of Alkenes
- 8.4 Hydrogenation of Alkenes
- 8.5 Halogenation of Alkenes
- 8.6 Dihydroxylation, Epoxidation, and Ozonolysis of Alkenes
- Problem Set
- Chapter 8 Videos
- Chapter 9: Alkynes Toggle Dropdown
- Chapter 10: Radicals Toggle Dropdown
- Chapter 11: Synthesis Toggle Dropdown
- Problem Sets Organic Chemistry I (CHEM 3401)
-
Chapter 12: Alcohols and Phenols
Toggle Dropdown
- 12.1 Alcohol Structure
- 12.2 Solubility
- 12.3 Boiling Point & Melting Point
- 12.4 Nomenclature
- 12.5 Alcohol Acidity
- 12.6 Reactions of Alcohols and Phenols
- 12.6.1 Substitution of the Hydroxyl Hydrogen
- 12.6.2 Nucleophilic Substitution of the Hydroxyl Group
- 12.6.3 Elimination Reactions of Alcohols
- 12.6.4 Oxidation Reactions of Alcohols
- 12.6.5 Reactions of Phenols
- 12.7 Practice Problems
- 12.7.1 Alcohol Nomenclature 1
- 12.7.2 Alcohol Nomenclature 2
- 12.7.3 Alcohol Nomenclature 3
- 12.7.4 Formation of Carbonyl Compounds
- 12.7.5 Functional Relationships of Alcohols
- 12.7.6 Reactions of Alcohols & Phenols
- 12.7.7 Alcohol Reactions
- Chapter 13: Ethers and Epoxides Toggle Dropdown
-
Chapter 14: Infrared Spectroscopy and Mass Spectrometry
Toggle Dropdown
- 14.1 Introduction fo Molecular Spectroscopy
- 14.2 Infrared Spectroscopy
- 14.2.1 Introduction
- 14.2.2 Vibrational Spectroscopy
- 14.2.3 Group Frequencies
- 14.2.4 Table of Characteristic IR Frequencies
- 14.3 Mass Spectrometry
- 14.3.1 The Mass Spectrometer
- 14.3.2 Characteristics of Mass Spectra
- 14.3.3 Isotopes
- 14.3.4 Fragmentation Patterns
- 14.3.5 High Resolution Spectra
- 14.3.6 MS Practice Problems
- 14.3.6a Problem 1
- 14.3.6b Problem 2
- 14.3.6c Problem 3
- 14.3.6d Problem 4
- 14.3.6e Problem 5
- 14.3.6f Problem 6
- 14.3.6g Problem 7
- 14.3.6h Problem 8
-
Chapter 15: Nuclear Magnetic Resonance Spectroscopy and UV-Visible Spectroscopy
Toggle Dropdown
- 15.1 Nuclear Magnetic Resonance Spectroscopy
- 15.1.1 Background
- 15.1.2 Proton NMR Spectroscopy
- 15.1.2a Introduction to Proton NMR Spectroscopy
- 15.1.2b Chemical Shift
- 15.1.2c Signal Strength
- 15.1.2d Hydroxyl Proton Exchange and the Influence of Hydrogen Bonding
- 15.1.2e Pi-Electron Functions
- 15.1.2f Solvent Effects
- 15.1.2g Spin-Spin Interactions
- 15.1.2h Examples
- 15.1.3 Carbon NMR Spectroscopy
- 15.1.4 NMR Practice Problems
- 15.1.4a Problem 1
- 15.1.4b Problem 2
- 15.1.4c Problem 3
- 15.1.4d Problem 4
- 15.1.4e Problem 5
- 15.1.4f Problem 6
- 15.1.4g Problem 7
- 15.1.4h Problem 8
- 15.1.4i Problem 9
- 15.1.4j Problem 10
- 15.1.5 Table of Proton NMR Shifts
- 15.1.6 Table of Carbon NMR Shifts
- 15.2 UV-Visible Spectroscopy
- 15.2.1 Background
- 15.2.2 The Electromagnetic Spectrum
- 15.2.3 UV-Visible Absorption Spectra
- 15.2.4 The Importance of Conjugation
- 15.3 Spectroscopy Practice Problems
- Chapter 16: Conjugated Pi Systems and Pericyclic Reactions Toggle Dropdown
-
Chapter 17: Aromatic Compounds
Toggle Dropdown
- 17.1 Aromaticity
- 17.1.1 Benzene
- 17.1.2 Fused Ring Compounds
- 17.1.3 Other Aromatic Compounds
- 17.1.4 Antiaromaticity
- 17.1.5 Practice Problems
- 17.1.5a Problem 1
- 17.1.5b Problem 2
- 17.2 Reactions of Substituent Groups
- 17.2.1 Oxidation of Alkyl Side-Chains
- 17.2.2 Bromination of Alkyl Side-Chains
- 17.2.3 Reduction of Nitro Groups
- Chapter 17 Videos
-
Chapter 18: Aromatic Substitution Reactions
Toggle Dropdown
- 18.1 Electrophilic Aromatic Substitution Reactions
- 18.2 Electrophilic Aromatic Substitution Mechanism
- 18.3 Electrophilic Aromatic Substitution Activation/Deactivation and Orientation
- 18.4 Electrophilic Substitution of Disubstituted Benzene Rings
- 18.5 Practice Problems
- 18.5.1 Problem 1
- 18.5.2 Problem 2
- 18.5.3 Problem 3
- 18.5.4 Problem 4
- 18.5.5 Problem 5
- 18.5.6 Problem 6
- 18.5.7 Problem 7
- Chapter 18 Videos
-
Chapter 19: Aldehydes and Ketones
Toggle Dropdown
- 19.1 Nomenclature
- 19.2 Preparation of Aldehydes and Ketones
- 19.3 Properties of Aldehydes and Ketones
- 19.4 Reactions of Aldehydes and Ketones
- 19.4.1 Addition Reactions
- 19.4.1a Hydration
- 19.4.1b Acetal Formation
- 19.4.1c Imine Formation
- 19.4.1d Cyanohydrin Formation
- 19.4.1e Hydride Reduction
- 19.4.1f Addition of Organometallic Reagents
- 19.4.2 Reduction of Aldehydes and Ketones
- 19.4.2a Wolff-Kishner Reduction
- 19.4.2b Clemmensen Reduction
- 19.4.3 Oxidation of Aldehydes and Ketones
- 19.5 Practice Problems
- 19.5.1 Problem 1
- 19.5.2 Problem 2
- 19.5.3 Problem 3
- 19.5.4 Problem 4
- 19.5.5 Problem 5
- 19.5.6 Problem 6
- 19.5.7 Problem 7
- 19.5.8 Problem 8
- 19.5.9 Problem 9
- 19.5.10 Problem 10
- 19.5.11 Problem 11
- 19.5.12 Problem 12
-
Chapter 20: Carboxylic Acids and Their Derivatives
- 20.1 Nomenclature
- 20.2 Physical Properties
- 20.3 Acidity
- 20.4 Preparation of Carboxylic Acids
- 20.5 Reactions of Carboxylic Acids
- 20.5.1 Salt Formation
- 20.5.2 Substitution of the Hydroxyl Hydrogen
- 20.5.3 Substitution of the Hydroxyl Group
- 20.5.4 Reduction
- 20.5.5 Oxidation
- 20.6 Practice Problems-Carboxylic Acids
- 20.6.1 Nomenclature Practice-1
- 20.6.2 Nomenclature Practice-2
- 20.6.3 Acidity
- 20.6.4 Reactions of Carboxylic Acids
- 20.7 Carboxylic Acid Derivatives
- 20.7.1 Related Derivatives
- 20.7.2 Nomenclature
- 20.7.3 Reactions
- 20.7.3a Acyl Substitution
- 20.7.3b Nitrile Hydrolysis
- 20.7.3c Reductions
- 20.7.3d Reactions with Organometallic Reagents
- 20.7.3e Dehydration of Amides
- 20.7.4 Practice Problems-Carboxylic Acid Derivatives
- 20.7.4a Nomenclature Practice-1
- 20.7.4b Nomenclature Practice-2
- 20.7.4c Carbonyl Compounds
- 20.8 Practice Problems
- 20.8.1 Problem 1
- 20.8.2 Problem 2
- 20.8.3 Problem 3
- 20.8.4 Problem 4
- 20.8.5 Problem 5
- 20.8.6 Problem 6
-
Chapter 21: Alpha Carbon Chemistry: Enols and Enolates
Toggle Dropdown
- 21.1 Reactions at the Alpha Carbon
- 21.2 Alpha Halogenation of Enols and Enolates
- 21.3 Aldol Reaction
- 21.4 Claisen Condensation
- 21.5 Alkylation at the Alpha Position
- 21.5.1 Enolate Alkylation
- 21.5.2 Dicarbonyl Alkylation
- 21.5.3 Decarboxylation Following Alkylation
- 21.5.4 Conjugate Reactions
- 21.5.4a Michael Reaction
- 21.5.4b Robinson Annulation
- 21.5.4c With Hydrides and Organometallics
- 21.6 Practice Problem
- 21.6.1 Problem 1
- Org Chem II - Problem Sets - Collection (CHEM 3402)
- Problem Set
20.7.3c Reductions
Reductions of carboxylic acid derivatives might be expected to lead either to aldehydes or alcohols, functional groups having a lower oxidation state of the carboxyl carbon. Indeed, it was noted earlier that carboxylic acids themselves are reduced to alcohols by lithium aluminum hydride. At this point it will be useful to consider three kinds of reductions:
![]() (i) catalytic hydrogenation
(i) catalytic hydrogenation
![]() (ii) complex metal hydride reductions
(ii) complex metal hydride reductions
![]() (iii) diborane reduction.
(iii) diborane reduction.
Catalytic Hydrogenation
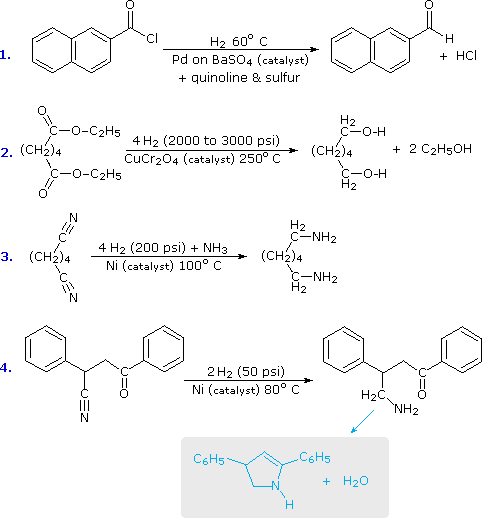
As a rule, the carbonyl group does not add hydrogen as readily as do the carbon-carbon double and triple bonds. Thus, it is fairly easy to reduce an alkene or alkyne function without affecting any carbonyl functions in the same molecule. By using a platinum catalyst and increased temperature and pressure, it is possible to reduce aldehydes and ketones to alcohols, but carboxylic acids, esters and amides are comparatively unreactive. The exceptional reactivity of acyl halides, on the other hand, facilitates their reduction under mild conditions, by using a poisoned palladium catalyst similar to that used for the partial reduction of alkynes to alkenes. This reduction stops at the aldehyde stage, providing us with a useful two-step procedure for converting carboxylic acids to aldehydes, as reaction #1 below demonstrates. Equivalent reductions of anhydrides have not been reported, but we might speculate that they would be reduced more easily than esters. The only other reduction of a carboxylic acid derivative that is widely used is that of nitriles to 1º-amines. Examples of these reductions are provided in the following diagram.
Complex Metal Hydride Reductions
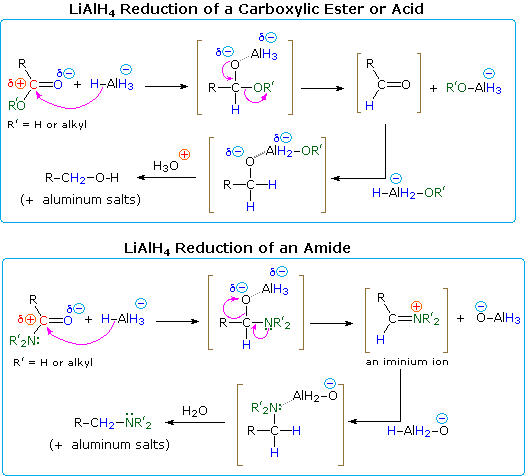
The use of lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4) as reagents for the reduction of aldehydes and ketones to 1º and 2º-alcohols respectively has been noted. Of these, lithium aluminum hydride, often abbreviated LAH, is the most useful for reducing carboxylic acid derivatives. Thanks to its high reactivity, LAH easily reduces all classes of carboxylic acid derivatives, generally to the –1 oxidation state. Acids, esters, anhydrides and acyl chlorides are all reduced to 1º-alcohols, and this method is superior to catalytic reduction in most cases. Since acyl chlorides and anhydrides are expensive and time consuming to prepare, acids and esters are the most commonly used reactants for this transformation. As in the reductions of aldehydes and ketones, the first step in each case is believed to be the irreversible addition of hydride to the electrophilic carbonyl carbon atom. Coordinative bonding of the carbonyl oxygen to a Lewis acidic metal (Li or Al) undoubtedly enhances that carbon's electrophilic character. This hydride addition is shown in the following diagrams, with the hydride-donating moiety being written as AlH4(–). All four hydrogens are potentially available to the reduction, but when carboxylic acids are reduced, one of the hydrides reacts with the acidic O–H to generate hydrogen gas. Although the lithium is not shown, it will be present in the products as a cationic component of ionic salts.
Amides are reduced to amines by treatment with LAH, and this has proven to be one of the most general methods for preparing all classes of amines (1º, 2º & 3º). Because the outcome of LAH reduction is so different for esters and amides, we must examine plausible reaction mechanisms for these reactions to discover a reason for this divergent behavior.
One explanation of the different course taken by the reductions of esters and amides lies in the nature of the different hetero atom substituents on the carbonyl group (colored green in the diagram). Nitrogen is more basic than oxygen, and amide anions are poorer leaving groups than alkoxide anions. Furthermore, oxygen forms especially strong bonds to aluminum. Addition of hydride produces a tetrahedral intermediate, shown in brackets, which has a polar oxygen-aluminum bond. Neither the hydrogen nor the alkyl group (R) is a possible leaving group, so if this tetrahedral species is to undergo an elimination to reform a hetero atom double bond, one of the two remaining substituents must be lost. For the ester this is an easy choice (described by the curved arrows). By eliminating an aluminum alkoxide (R'O–Al), an aldehyde is formed, and this is quickly reduced to the salt of a 1º-alcohol by LAH. In the case of the amide, aldehyde formation requires the loss of an aluminum amide (R'2N–Al), an unlikely process. Alternatively, the more basic nitrogen may act to eject a metal oxide species (e.g. Al–O(–)), and the resulting iminium double bond would then be reduced to an amine. This is the course followed by most amide reductions; but in the case of 1º-amides, the acidity of the nitrogen hydrogens coupled with the basicity of hydride enables a facile elimination of the oxygen (as an oxide moiety). The resulting nitrile intermediate is then reduced to a 1º-amine. Nitriles are in fact a major product when less than a full equivalency of LiAlH4 is used. A mechanism will be shown above by clicking on the diagram.
Lithium aluminum hydride reduces nitriles to 1º-amines, as shown in the following equation. An initial hydride addition to the electrophilic nitrile carbon atom generates the salt of an imine intermediate. This is followed by a second hydride transfer, and the resulting metal amine salt is hydrolyzed to a 1º-amine. This method provides a useful alternative to the catalytic reduction of nitriles, described above, when alkene or alkyne functions are present.
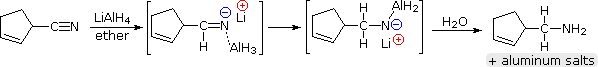
In contrast to the usefulness of lithium aluminum hydride in reducing various carboxylic acid derivatives, sodium borohydride is seldom chosen for this purpose. First, NaBH4 is often used in hydroxylic solvents (water and alcohols), and these would react with acyl chlorides and anhydrides. Furthermore, it is sparingly soluble in relatively nonpolar solvents, particularly at low temperatures. Second, NaBH4 is much less reactive than LAH, failing to reduce amides and acids (they form carboxylate salts) at all, and reducing esters very slowly.
Since relatively few methods exist for the reduction of carboxylic acid derivatives to aldehydes, it would be useful to modify the reactivity and solubility of LAH to permit partial reductions of this kind to be achieved. The most fruitful approach to this end has been to attach alkoxy or alkyl groups on the aluminum. This not only modifies the reactivity of the reagent as a hydride donor, but also increases its solubility in nonpolar solvents. Two such reagents will be mentioned here; the reactive hydride atom is colored blue.
![]() Lithium tri-tert-butoxyaluminohydride (LtBAH), LiAl[OC(CH3)3]3H : Soluble in THF, diglyme & ether.
Lithium tri-tert-butoxyaluminohydride (LtBAH), LiAl[OC(CH3)3]3H : Soluble in THF, diglyme & ether.
![]() Diisobutylaluminum hydride (DIBAH), [(CH3)2CHCH2]2AlH : Soluble in toluene, THF & ether.
Diisobutylaluminum hydride (DIBAH), [(CH3)2CHCH2]2AlH : Soluble in toluene, THF & ether.
Each of these reagents carries one equivalent of hydride. The first (LtBAH) is a complex metal hydride, but the second is simply an alkyl derivative of aluminum hydride. In practice, both reagents are used in equimolar amounts, and usually at temperatures well below 0 ºC. The following examples illustrate how aldehydes may be prepared from carboxylic acid derivatives by careful application of these reagents. A temperature of -78 ºC is easily maintained by using dry-ice as a coolant. The reduced intermediates that lead to aldehydes will be displayed on clicking the "Show Intermediates" button. With excess reagent at temperatures above 0 ºC most carboxylic acid derivatives are reduced to alcohols or amines.
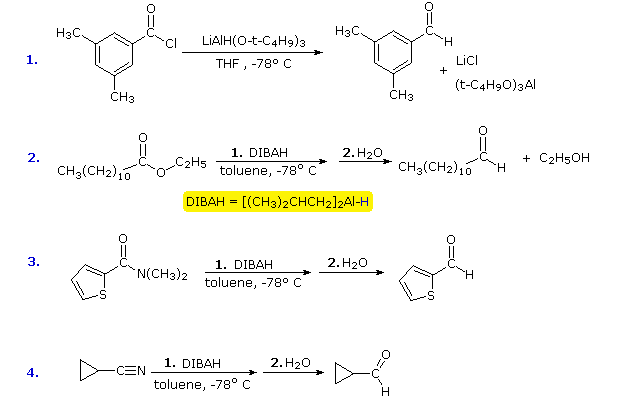
The second and third equations illustrate the extreme difference in hydrogenation reactivity between esters and nitriles. This is further demonstrated by the last reaction, in which a nitrile is preferentially reduced in the presence of a carbonyl group and two benzene rings. The resulting 1º-amine immediately reacts with the carbonyl function to give a cyclic enamine product (colored light blue).
In most nitrile reductions ammonia is added to inhibit the formation of a 2º-amine by-product. This may occur by way of an intermediate aldehyde imine created by addition of the first equivalent of hydrogen. The following equations show how such an imine species might react with the 1º-amine product to give a substituted imine (2nd equation), which would then add hydrogen to generate a 2º-amine. Excess ammonia shifts the imine equilibrium to the left, as written below.
| (1) | R–C≡N + H2 |
catalyst |
RCH=NH imine |
H2 |
RCH2NH2 1º-amine |
|---|
| (2) | RCH=NH + RCH2NH2 imine 1º-amine |
 |
RCH=NCH2R + NH3 substituted imine |
H2 & catalyst |
RCH2NHCH2R 2º-amine |
|---|
Diborane, B2H6
The reducing characteristics of diborane (disassociated to BH3 in ether or THF solution) were first introduced as addition reactions to alkenes and alkynes. This remains a primary application of this reagent, but it also effects rapid and complete reduction of carboxylic acids, amides and nitriles. Other than LAH, this reagent provides one of the best methods for reducing carboxylic acids to 1º-alcohols.
(1) |
R–CO2H + BH3 |
ether soln. |
[RCH2O–B] |
H2O2 |
RCH2–OH |
|---|
(2) |
R–C≡N + BH3 |
ether soln. |
RCH2–NH–B |
H2O |
RCH2–NH2 |
|---|
Overview of Reducing Agents
The following table summarizes the influence each of the reducing systems discussed above has on the different classes of carboxylic acid derivatives. Note that LAH is the strongest reducing agent listed, and it reduces all the substrates. In a similar sense, acyl chlorides are the most reactive substrate. They are reduced by all the reagents, but only a few of these provide synthetically useful transformations.
| Function Reagent |
Aldehydes |
Carboxylic |
Carboxylic |
Acyl |
Amides |
Nitriles |
|---|---|---|---|---|---|---|
|
H2 |
alcohols ( slow, Pt, Pd ) |
(v. slow) | (v. slow) | aldehydes ( Pd/BaSO4) |
(v. slow) | amines ( Ni cat. ) |
|
NaBH4 |
alcohols | N.R. | alcohols (slow) |
complex mixture |
N.R. | N.R. |
|
LiAlH4 |
alcohols | 1º-alcohol | alcohols | 1º-alcohol | amines | 1º-amine |
|
LiAlH(Ot-Bu)3 |
alcohols (slow at 0º) |
N.R. | v. slow | aldehyde (-78 º C) |
aldehyde (-78 º C) |
aldehyde (0 º C) |
|
(iso-Bu)2AlH |
alcohols | 1º-alcohol | aldehyde (-78º C) |
1º-alcohol | aldehyde (-78 º C) |
aldehyde (-78 º C) |
|
B2H6 |
alcohols (slow)
|
1º-alcohol | (v. slow) | complex mixture |
1º-amine | 1º-amine |
|
Color Code |
||||||
| Reduction occurs readily under normal conditions of temperature and pressure. | ||||||
| Reduction occurs readily, but selectivity requires low temperature. | ||||||
| Slow reduction occurs. Heating and/or high pressures of hydrogen are needed for effective use. | ||||||
| Reduction occurs very slowly or not at all (N.R.). | ||||||