Organic Chemistry Text Book (CHEM 3401 and 3402)
- Home
- Chapter 1: A Review of General ChemistryToggle Dropdown
- 1.1 Introduction to Organic Chemistry
- 1.2 Electrons, Bonds, and Lewis Structures
- 1.3 Identifying Formal Charges
- 1.4 Atomic Orbitals
- 1.5 Valence Bond Theory
- 1.6 Molecular Orbital Theory/Hybridization
- 1.7 VSEPR Theory: Predicting Geometry
- 1.8 Dipole Moments and Molecular Polarity
- 1.9 Intermolecular Forces and Physical Properties
- Problem Set
- Videos for chapter 1
- Chapter 2: Molecular RepresentationsToggle Dropdown
- Chapter 3: Acids and BasesToggle Dropdown
- Chapter 4: Alkanes and CycloalkanesToggle Dropdown
- Chapter 5: StereochemistryToggle Dropdown
- Chapter 6: Chemical Reactivity and MechanismsToggle Dropdown
- Chapter 7: Substitution ReactionsToggle Dropdown
- Chapter 8: Addition Reactions of AlkenesToggle Dropdown
- 8.1 Introduction of Addition Reactions
- 8.1 Nomenclature of Alkenes
- 8.2 Hydrohalogenation of Alkenes
- 8.3 Hydration, Hydroboration, and Oxymercuration of Alkenes
- 8.4 Hydrogenation of Alkenes
- 8.5 Halogenation of Alkenes
- 8.6 Dihydroxylation, Epoxidation, and Ozonolysis of Alkenes
- Problem Set
- Chapter 8 Videos
- Chapter 9: Alkynes
- Chapter 10: RadicalsToggle Dropdown
- Chapter 11: SynthesisToggle Dropdown
- Problem Sets Organic Chemistry I (CHEM 3401)
- Chapter 12: Alcohols and PhenolsToggle Dropdown
- 12.1 Alcohol Structure
- 12.2 Solubility
- 12.3 Boiling Point & Melting Point
- 12.4 Nomenclature
- 12.5 Alcohol Acidity
- 12.6 Reactions of Alcohols and Phenols
- 12.6.1 Substitution of the Hydroxyl Hydrogen
- 12.6.2 Nucleophilic Substitution of the Hydroxyl Group
- 12.6.3 Elimination Reactions of Alcohols
- 12.6.4 Oxidation Reactions of Alcohols
- 12.6.5 Reactions of Phenols
- 12.7 Practice Problems
- 12.7.1 Alcohol Nomenclature 1
- 12.7.2 Alcohol Nomenclature 2
- 12.7.3 Alcohol Nomenclature 3
- 12.7.4 Formation of Carbonyl Compounds
- 12.7.5 Functional Relationships of Alcohols
- 12.7.6 Reactions of Alcohols & Phenols
- 12.7.7 Alcohol Reactions
- Chapter 13: Ethers and EpoxidesToggle Dropdown
- Chapter 14: Infrared Spectroscopy and Mass SpectrometryToggle Dropdown
- 14.1 Introduction fo Molecular Spectroscopy
- 14.2 Infrared Spectroscopy
- 14.2.1 Introduction
- 14.2.2 Vibrational Spectroscopy
- 14.2.3 Group Frequencies
- 14.2.4 Table of Characteristic IR Frequencies
- 14.3 Mass Spectrometry
- 14.3.1 The Mass Spectrometer
- 14.3.2 Characteristics of Mass Spectra
- 14.3.3 Isotopes
- 14.3.4 Fragmentation Patterns
- 14.3.5 High Resolution Spectra
- 14.3.6 MS Practice Problems
- 14.3.6a Problem 1
- 14.3.6b Problem 2
- 14.3.6c Problem 3
- 14.3.6d Problem 4
- 14.3.6e Problem 5
- 14.3.6f Problem 6
- 14.3.6g Problem 7
- 14.3.6h Problem 8
- Chapter 15: Nuclear Magnetic Resonance Spectroscopy and UV-Visible SpectroscopyToggle Dropdown
- 15.1 Nuclear Magnetic Resonance Spectroscopy
- 15.1.1 Background
- 15.1.2 Proton NMR Spectroscopy
- 15.1.2a Introduction to Proton NMR Spectroscopy
- 15.1.2b Chemical Shift
- 15.1.2c Signal Strength
- 15.1.2d Hydroxyl Proton Exchange and the Influence of Hydrogen Bonding
- 15.1.2e Pi-Electron Functions
- 15.1.2f Solvent Effects
- 15.1.2g Spin-Spin Interactions
- 15.1.2h Examples
- 15.1.3 Carbon NMR Spectroscopy
- 15.1.4 NMR Practice Problems
- 15.1.4a Problem 1
- 15.1.4b Problem 2
- 15.1.4c Problem 3
- 15.1.4d Problem 4
- 15.1.4e Problem 5
- 15.1.4f Problem 6
- 15.1.4g Problem 7
- 15.1.4h Problem 8
- 15.1.4i Problem 9
- 15.1.4j Problem 10
- 15.1.5 Table of Proton NMR Shifts
- 15.1.6 Table of Carbon NMR Shifts
- 15.2 UV-Visible Spectroscopy
- 15.2.1 Background
- 15.2.2 The Electromagnetic Spectrum
- 15.2.3 UV-Visible Absorption Spectra
- 15.2.4 The Importance of Conjugation
- 15.3 Spectroscopy Practice Problems
- Chapter 16: Conjugated Pi Systems and Pericyclic ReactionsToggle Dropdown
- Chapter 17: Aromatic CompoundsToggle Dropdown
- 17.1 Aromaticity
- 17.1.1 Benzene
- 17.1.2 Fused Ring Compounds
- 17.1.3 Other Aromatic Compounds
- 17.1.4 Antiaromaticity
- 17.1.5 Practice Problems
- 17.1.5a Problem 1
- 17.1.5b Problem 2
- 17.2 Reactions of Substituent Groups
- 17.2.1 Oxidation of Alkyl Side-Chains
- 17.2.2 Bromination of Alkyl Side-Chains
- 17.2.3 Reduction of Nitro Groups
- Chapter 17 Videos
- Chapter 18: Aromatic Substitution ReactionsToggle Dropdown
- 18.1 Electrophilic Aromatic Substitution Reactions
- 18.2 Electrophilic Aromatic Substitution Mechanism
- 18.3 Electrophilic Aromatic Substitution Activation/Deactivation and Orientation
- 18.4 Electrophilic Substitution of Disubstituted Benzene Rings
- 18.5 Practice Problems
- 18.5.1 Problem 1
- 18.5.2 Problem 2
- 18.5.3 Problem 3
- 18.5.4 Problem 4
- 18.5.5 Problem 5
- 18.5.6 Problem 6
- 18.5.7 Problem 7
- Chapter 18 Videos
- Chapter 19: Aldehydes and KetonesToggle Dropdown
- 19.1 Nomenclature
- 19.2 Preparation of Aldehydes and Ketones
- 19.3 Properties of Aldehydes and Ketones
- 19.4 Reactions of Aldehydes and Ketones
- 19.4.1 Addition Reactions
- 19.4.1a Hydration
- 19.4.1b Acetal Formation
- 19.4.1c Imine Formation
- 19.4.1d Cyanohydrin Formation
- 19.4.1e Hydride Reduction
- 19.4.1f Addition of Organometallic Reagents
- 19.4.2 Reduction of Aldehydes and Ketones
- 19.4.2a Wolff-Kishner Reduction
- 19.4.2b Clemmensen Reduction
- 19.4.3 Oxidation of Aldehydes and Ketones
- 19.5 Practice Problems
- 19.5.1 Problem 1
- 19.5.2 Problem 2
- 19.5.3 Problem 3
- 19.5.4 Problem 4
- 19.5.5 Problem 5
- 19.5.6 Problem 6
- 19.5.7 Problem 7
- 19.5.8 Problem 8
- 19.5.9 Problem 9
- 19.5.10 Problem 10
- 19.5.11 Problem 11
- 19.5.12 Problem 12
- Chapter 20: Carboxylic Acids and Their DerivativesToggle Dropdown
- 20.1 Nomenclature
- 20.2 Physical Properties
- 20.3 Acidity
- 20.4 Preparation of Carboxylic Acids
- 20.5 Reactions of Carboxylic Acids
- 20.5.1 Salt Formation
- 20.5.2 Substitution of the Hydroxyl Hydrogen
- 20.5.3 Substitution of the Hydroxyl Group
- 20.5.4 Reduction
- 20.5.5 Oxidation
- 20.6 Practice Problems-Carboxylic Acids
- 20.6.1 Nomenclature Practice-1
- 20.6.2 Nomenclature Practice-2
- 20.6.3 Acidity
- 20.6.4 Reactions of Carboxylic Acids
- 20.7 Carboxylic Acid Derivatives
- 20.7.1 Related Derivatives
- 20.7.2 Nomenclature
- 20.7.3 Reactions
- 20.7.3a Acyl Substitution
- 20.7.3b Nitrile Hydrolysis
- 20.7.3c Reductions
- 20.7.3d Reactions with Organometallic Reagents
- 20.7.3e Dehydration of Amides
- 20.7.4 Practice Problems-Carboxylic Acid Derivatives
- 20.7.4a Nomenclature Practice-1
- 20.7.4b Nomenclature Practice-2
- 20.7.4c Carbonyl Compounds
- 20.8 Practice Problems
- 20.8.1 Problem 1
- 20.8.2 Problem 2
- 20.8.3 Problem 3
- 20.8.4 Problem 4
- 20.8.5 Problem 5
- 20.8.6 Problem 6
- Chapter 21: Alpha Carbon Chemistry: Enols and EnolatesToggle Dropdown
- 21.1 Reactions at the Alpha Carbon
- 21.2 Alpha Halogenation of Enols and Enolates
- 21.3 Aldol Reaction
- 21.4 Claisen Condensation
- 21.5 Alkylation at the Alpha Position
- 21.5.1 Enolate Alkylation
- 21.5.2 Dicarbonyl Alkylation
- 21.5.3 Decarboxylation Following Alkylation
- 21.5.4 Conjugate Reactions
- 21.5.4a Michael Reaction
- 21.5.4b Robinson Annulation
- 21.5.4c With Hydrides and Organometallics
- 21.6 Practice Problem
- 21.6.1 Problem 1
- Org Chem II - Problem Sets - Collection (CHEM 3402)
- Problem Set
9.5 Addition Reactions of Alkynes
Addition Reactions of Alkynes
A carbon-carbon triple bond may be located at any unbranched site within a carbon chain or at the end of a chain, in which case it is called terminal. Because of its linear configuration ( the bond angle of a sp-hybridized carbon is 180º ), a ten-membered carbon ring is the smallest that can accommodate this function without excessive strain. Since the most common chemical transformation of a carbon-carbon double bond is an addition reaction, we might expect the same to be true for carbon-carbon triple bonds. Indeed, most of the alkene addition reactionsdiscussed earlier also take place with alkynes, and with similar regio- and stereoselectivity.
1. Catalytic Hydrogenation
The catalytic addition of hydrogen to 2-butyne not only serves as an example of such an addition reaction, but also provides heat of reaction data that reflect the relative thermodynamic stabilities of these hydrocarbons, as shown in the diagram to the right. 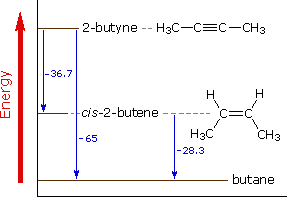 From the heats of hydrogenation, shown in blue in units of kcal/mole, it would appear that alkynes are thermodynamically less stable than alkenes to a greater degree than alkenes are less stable than alkanes. The standard bond energies for carbon-carbon bonds confirm this conclusion. Thus, a double bond is stronger than a single bond, but not twice as strong. The difference ( 63 kcal/mole ) may be regarded as the strength of the π-bond component. Similarly, a triple bond is stronger than a double bond, but not 50% stronger. Here the difference ( 54 kcal/mole ) may be taken as the strength of the second π-bond. The 9 kcal/mole weakening of this second π-bond is reflected in the heat of hydrogenation numbers ( 36.7 - 28.3 = 8.4 ).
From the heats of hydrogenation, shown in blue in units of kcal/mole, it would appear that alkynes are thermodynamically less stable than alkenes to a greater degree than alkenes are less stable than alkanes. The standard bond energies for carbon-carbon bonds confirm this conclusion. Thus, a double bond is stronger than a single bond, but not twice as strong. The difference ( 63 kcal/mole ) may be regarded as the strength of the π-bond component. Similarly, a triple bond is stronger than a double bond, but not 50% stronger. Here the difference ( 54 kcal/mole ) may be taken as the strength of the second π-bond. The 9 kcal/mole weakening of this second π-bond is reflected in the heat of hydrogenation numbers ( 36.7 - 28.3 = 8.4 ).
Since alkynes are thermodynamically less stable than alkenes, we might expect addition reactions of the former to be more exothermic and relatively faster than equivalent reactions of the latter. In the case of catalytic hydrogenation, the usual Pt and Pd hydrogenation catalysts are so effective in promoting addition of hydrogen to both double and triple carbon-carbon bonds that the alkene intermediate formed by hydrogen addition to an alkyne cannot be isolated. A less efficient catalyst, Lindlar's catalyst, prepared by deactivating (or poisoning) a conventional palladium catalyst by treating it with lead acetate and quinoline, permits alkynes to be converted to alkenes without further reduction to an alkane. The addition of hydrogen is stereoselectively syn (e.g. 2-butyne gives cis-2-butene). A complementary stereoselective reduction in the anti mode may be accomplished by a solution of sodium in liquid ammonia. This reaction will be discussed later in this section.
| R-C≡C-R + H2 & Lindlar catalyst ——> cis R-CH=CH-R |
| R-C≡C-R + 2 Na in NH3 (liq) ——> trans R-CH=CH-R + 2 NaNH2 |
Alkenes and alkynes show a curious difference in behavior toward catalytic hydrogenation. Independent studies of hydrogenation rates for each class indicate that alkenes react more rapidly than alkynes. However, careful hydrogenation of an alkyne proceeds exclusively to the alkene until the former is consumed, at which point the product alkene is very rapidly hydrogenated to an alkane. This behavior is nicely explained by differences in the stages of the hydrogenation reaction. Before hydrogen can add to a multiple bond the alkene or alkyne must be adsorbed on the catalyst surface. In this respect, the formation of stable platinum (and palladium) complexes with alkenes has been described earlier. Since alkynes adsorb more strongly to such catalytic surfaces than do alkenes, they preferentially occupy reactive sites on the catalyst. Subsequent transfer of hydrogen to the adsorbed alkyne proceeds slowly, relative to the corresponding hydrogen transfer to an adsorbed alkene molecule. Consequently, reduction of triple bonds occurs selectively at a moderate rate, followed by rapid addition of hydrogen to the alkene product. The Lindlar catalyst permits adsorption and reduction of alkynes, but does not adsorb alkenes sufficiently to allow their reduction.
2. Addition by Electrophilic Reagents
When the addition reactions of electrophilic reagents, such as strong Brønsted acids and halogens, to alkynes are studied we find a curious paradox. The reactions are even more exothermic than the additions to alkenes, and yet the rate of addition to alkynes is slower by a factor of 100 to 1000 than addition to equivalently substituted alkenes. The reaction of one equivalent of bromine with 1-penten-4-yne, for example, gave 4,5-dibromo-1-pentyne as the chief product.
Although these electrophilic additions to alkynes are sluggish, they do take place and generally display Markovnikov Rule regioselectivity and anti-stereoselectivity. One problem, of course, is that the products of these additions are themselves substituted alkenes and can therefore undergo further addition. Because of their high electronegativity, halogen substituents on a double bond act to reduce its nucleophilicity, and thereby decrease the rate of electrophilic addition reactions. Consequently, there is a delicate balance as to whether the product of an initial addition to an alkyne will suffer further addition to a saturated product. Although the initial alkene products can often be isolated and identified, they are commonly present in mixtures of products and may not be obtained in high yield. The following reactions illustrate many of these features. In the last example, 1,2-diodoethene does not suffer further addition inasmuch as vicinal-diiodoalkanes are relatively unstable.
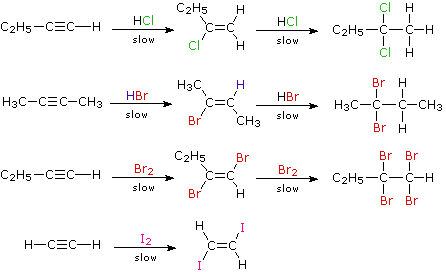
As a rule, electrophilic addition reactions to alkenes and alkynes proceed by initial formation of a pi-complex, in which the electrophile accepts electrons from and becomes weakly bonded to the multiple bond. Such complexes are formed reversibly and may then reorganize to a reactive intermediate in a slower, rate-determining step. Reactions with alkynes are more sensitive to solvent changes and catalytic influences than are equivalent alkenes. For examples and a discussion of mechanisms click here.
Why are the reactions of alkynes with electrophilic reagents more sluggish than the corresponding reactions of alkenes? After all, addition reactions to alkynes are generally more exothermic than additions to alkenes, and there would seem to be a higher π-electron density about the triple bond ( two π-bonds versus one ). Two factors are significant in explaining this apparent paradox. First, although there are more π-electrons associated with the triple bond, the sp-hybridized carbons exert a strong attraction for these π-electrons, which are consequently bound more tightly to the functional group than are the π-electrons of a double bond. This is seen in the ionization potentials of ethylene and acetylene.
| Acetylene | HC≡CH + Energy ——> [HC≡CH •(+) + e(–) | ΔH = +264 kcal/mole | |
|---|---|---|---|
| Ethylene | H2C=CH2 + Energy ——> [H2C=CH2] •(+) + e(–) | ΔH = +244 kcal/mole | |
| Ethane | H3C–CH3 + Energy ——> [H3C–CH3] •(+) + e(–) | ΔH = +296 kcal/mole |
As defined by the preceding equations, an ionization potential is the minimum energy required to remove an electron from a molecule of a compound. Since pi-electrons are less tightly held than sigma-electrons, we expect the ionization potentials of ethylene and acetylene to be lower than that of ethane, as is the case. Gas-phase proton affinities show the same order, with ethylene being more basic than acetylene, and ethane being less basic than either. Since the initial interaction between an electrophile and an alkene or alkyne is the formation of a pi-complex, in which the electrophile accepts electrons from and becomes weakly bonded to the multiple bond, the relatively slower reactions of alkynes becomes understandable.
A second factor is presumed to be the stability of the carbocation intermediate generated by sigma-bonding of a proton or other electrophile to one of the triple bond carbon atoms. This intermediate has its positive charge localized on an unsaturated carbon, and such vinyl cations are less stable than their saturated analogs. Indeed, we can modify our earlier ordering of carbocation stability to include these vinyl cations in the manner shown below. It is possible that vinyl cations stabilized by conjugation with an aryl substituent are intermediates in HX addition to alkynes of the type Ar-C≡C-R, but such intermediates are not formed in all alkyne addition reactions.
|
Application of the Hammond postulate indicates that the activation energy for the generation of a vinyl cation intermediate would be higher than that for a lower energy intermediate. This is illustrated for alkenes versus alkynes by the following energy diagrams.
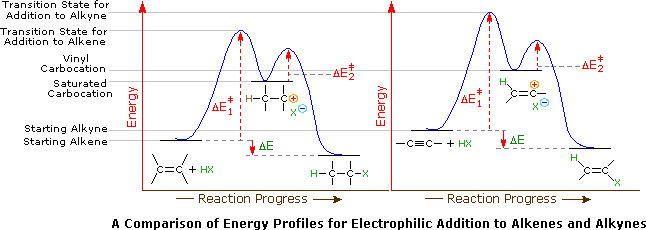
Despite these differences, electrophilic additions to alkynes have emerged as exceptionally useful synthetic transforms. For example, addition of HCl, acetic acid and hydrocyanic acid to acetylene give respectively the useful monomers vinyl chloride, vinyl acetate and acrylonitrile, as shown in the following equations. Note that in these and many other similar reactions transition metals, such as copper and mercury salts, are effective catalysts.
| HC≡CH + HCl + HgCl2 (on carbon) ——> H2C=CHCl vinyl chloride |
| HC≡CCH2Cl + HCl + HgCl2 ——> H2C=CClCH2Cl 2,3-dichloropropene |
| HC≡CH + CH3CO2H + HgSO4 ——> H2C=CHOCOCH3 vinyl acetate |
| HC≡CH + HCN + Cu2Cl2 ——> H2C=CHCN acryonitrile |
Complexes formed by alkenes and alkynes with transition metals are different from the simple pi-complexes noted above. Here a synergic process involving donation of electrons from a filled π-orbital of the organic ligand into an empty d-orbital of the metal, together with back-donation of electrons from another d-orbital of the metal into the empty π*-antibonding orbital of the ligand. A model of a Pt(II) complex with acetylene may be viewed by clicking here
3. Hydration of Alkynes and Tautomerism
As with alkenes, the addition of water to alkynes requires a strong acid, usually sulfuric acid, and is facilitated by mercuric sulfate. However, unlike the additions to double bonds which give alcohol products, addition of water to alkynes gives ketone products ( except for acetylene which yields acetaldehyde ). The explanation for this deviation lies in enol-keto tautomerization, illustrated by the following equation. The initial product from the addition of water to an alkyne is an enol (a compound having a hydroxyl substituent attached to a double-bond), and this immediately rearranges to the more stable keto tautomer.
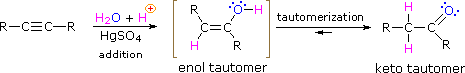
Tautomers are defined as rapidly interconverted constitutional isomers, usually distinguished by a different bonding location for a labile hydrogen atom (colored red here) and a differently located double bond. The equilibrium between tautomers is not only rapid under normal conditions, but it often strongly favors one of the isomers ( acetone, for example, is 99.999% keto tautomer ). Even in such one-sided equilibria, evidence for the presence of the minor tautomer comes from the chemical behavior of the compound. Tautomeric equilibria are catalyzed by traces of acids or bases that are generally present in most chemical samples. The three examples shown below illustrate these reactions for different substitutions of the triple-bond. The tautomerization step is indicated by a red arrow. For terminal alkynes the addition of water follows the Markovnikov rule, as in the second example below, and the final product ia a methyl ketone ( except for acetylene, shown in the first example ). For internal alkynes ( the triple-bond is within a longer chain ) the addition of water is not regioselective. If the triple-bond is not symmetrically located ( i.e. if R & R' in the third equation are not the same ) two isomeric ketones will be formed.
| HC≡CH + H2O + HgSO4 & H2SO4 ——> [ H2C=CHOH ] ——> H3C-CH=O |
| RC≡CH + H2O + HgSO4 & H2SO4 ——> [ RC(OH)=CH2 ] ——> RC(=O)CH3 |
| RC≡CR' + H2O + HgSO4 & H2SO4 ——> [ RHC=C(OH)R' + RC(OH)=CHR' ] ——> RCH2-C(=O)R' + RC(=O)-CH2R' |
Two factors have an important influence on the enol-keto tautomerizations described here. The first is the potential energy difference between the tautomeric isomers. This factor determines the position of the equilibrium state. The second factor is the activation energy for the interconversion of one tautomer to the other. This factor determines the rate of rearrangement. Since the potential energy or stability of a compound is in large part a function of its covalent bond energies, we can estimate the relative energy of keto and enol tautomers by considering the bonds that are changed in the rearrangement. From the following diagram, we see that only three significant changes occur, and the standard bond energies for those changes are given to the right of the equation. The keto tautomer has a 17.5 kcal/mole advantage in bond energy, so its predominance at equilibrium is expected.
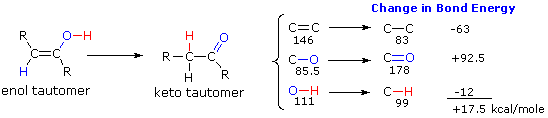
The rapidity with which enol-keto tautomerization occurs suggests that the activation energy for this process is low. We have noted that the rearrangement is acid & base catalyzed, and very careful experiments have shown that interconversion of tautomers is much slower if such catalysts are absent. A striking example of the influence of activation energy on such transformations may be seen in the following hypothetical rearrangement. Here we have substituted a methyl group (colored maroon) for the proton of a conventional tautomerism, and the methyl shifts from oxygen to carbon just as the proton does in going from an enol to a ketone.
H2C=CH-O-CH3 –X–> CH3-CH2-CH=O
The potential energy change for this rearrangement is even more advantageous than for enol-keto tautomerism, being estimated at over 25 kcal/mole from bond energy changes. Despite this thermodynamic driving force, the enol ether described above is completely stable to base treatment, and undergoes rapid acid-catalyzed hydrolysis with loss of methanol, rather than rearrangement. The controlling difference in this case must be a prohibitively high activation energy for the described rearrangement, combined with lower energy alternative reaction paths.
4. Hydroboration Reactions
Diborane reacts readily with alkynes, but the formation of substituted alkene products leaves open the possibility of a second addition reaction. A clever technique for avoiding this event takes advantage of the fact that alkynes do not generally suffer from steric hindrance near the triple-bond (the configuration of this functional group is linear). Consequently, large or bulky electrophilic reagents add easily to the triple-bond, but the resulting alkene is necessarily more crowded or sterically hindered and resists further additions. The bulky hydroboration reagent needed for this strategy is prepared by reaction of diborane with 2-methyl-2-butene, a highly branched alkene. Because of the alkyl branching, only two alkenes add to a BH3 moiety (steric hindrance again), leaving one B-H covalent bond available for reaction with an alkyne, as shown below. The resulting dialkyl borane is called disiamylborane, a contraction of di-secondary-isoamylborane (amyl is an old name for pentyl).
An important application of disiamylborane is its addition reaction to terminal alkynes. As with alkenes, the B-H reagent group adds in an apparently anti-Markovnikov manner, due to the fact that the boron is the electrophile, not the hydrogen. Further addition to the resulting boron-substituted alkene does not occur, and the usual oxidative removal of boron by alkaline hydrogen peroxide gives an enol which rapidly rearranges to the aldehyde tautomer. Thus, by the proper choice of reagents, terminal alkynes may be converted either to methyl ketones (mercuric ion catalyzed hydration) or aldehydes (hydroboration followed by oxidation).
Hydroboration of internal alkynes is not a particularly useful procedure because a mixture of products will often be obtained, unless the triple-bond is symmetrically substituted. Mercuric ion catalyzed hydration gives similar results.
5. Oxidations
Reactions of alkynes with oxidizing agents such as potassium permanganate and ozone usually result in cleavage of the triple-bond to give carboxylic acid products. A general equation for this kind of transformation follows. The symbol [O] is often used in a general way to denote an oxidation.
