Organic Chemistry Text Book (CHEM 3401 and 3402)
- Home
- Chapter 1: A Review of General ChemistryToggle Dropdown
- 1.1 Introduction to Organic Chemistry
- 1.2 Electrons, Bonds, and Lewis Structures
- 1.3 Identifying Formal Charges
- 1.4 Atomic Orbitals
- 1.5 Valence Bond Theory
- 1.6 Molecular Orbital Theory/Hybridization
- 1.7 VSEPR Theory: Predicting Geometry
- 1.8 Dipole Moments and Molecular Polarity
- 1.9 Intermolecular Forces and Physical Properties
- Problem Set
- Videos for chapter 1
- Chapter 2: Molecular RepresentationsToggle Dropdown
- Chapter 3: Acids and BasesToggle Dropdown
- Chapter 4: Alkanes and Cycloalkanes
- Chapter 5: StereochemistryToggle Dropdown
- Chapter 6: Chemical Reactivity and MechanismsToggle Dropdown
- Chapter 7: Substitution ReactionsToggle Dropdown
- Chapter 8: Addition Reactions of AlkenesToggle Dropdown
- 8.1 Introduction of Addition Reactions
- 8.1 Nomenclature of Alkenes
- 8.2 Hydrohalogenation of Alkenes
- 8.3 Hydration, Hydroboration, and Oxymercuration of Alkenes
- 8.4 Hydrogenation of Alkenes
- 8.5 Halogenation of Alkenes
- 8.6 Dihydroxylation, Epoxidation, and Ozonolysis of Alkenes
- Problem Set
- Chapter 8 Videos
- Chapter 9: AlkynesToggle Dropdown
- Chapter 10: RadicalsToggle Dropdown
- Chapter 11: SynthesisToggle Dropdown
- Problem Sets Organic Chemistry I (CHEM 3401)
- Chapter 12: Alcohols and PhenolsToggle Dropdown
- 12.1 Alcohol Structure
- 12.2 Solubility
- 12.3 Boiling Point & Melting Point
- 12.4 Nomenclature
- 12.5 Alcohol Acidity
- 12.6 Reactions of Alcohols and Phenols
- 12.6.1 Substitution of the Hydroxyl Hydrogen
- 12.6.2 Nucleophilic Substitution of the Hydroxyl Group
- 12.6.3 Elimination Reactions of Alcohols
- 12.6.4 Oxidation Reactions of Alcohols
- 12.6.5 Reactions of Phenols
- 12.7 Practice Problems
- 12.7.1 Alcohol Nomenclature 1
- 12.7.2 Alcohol Nomenclature 2
- 12.7.3 Alcohol Nomenclature 3
- 12.7.4 Formation of Carbonyl Compounds
- 12.7.5 Functional Relationships of Alcohols
- 12.7.6 Reactions of Alcohols & Phenols
- 12.7.7 Alcohol Reactions
- Chapter 13: Ethers and EpoxidesToggle Dropdown
- Chapter 14: Infrared Spectroscopy and Mass SpectrometryToggle Dropdown
- 14.1 Introduction fo Molecular Spectroscopy
- 14.2 Infrared Spectroscopy
- 14.2.1 Introduction
- 14.2.2 Vibrational Spectroscopy
- 14.2.3 Group Frequencies
- 14.2.4 Table of Characteristic IR Frequencies
- 14.3 Mass Spectrometry
- 14.3.1 The Mass Spectrometer
- 14.3.2 Characteristics of Mass Spectra
- 14.3.3 Isotopes
- 14.3.4 Fragmentation Patterns
- 14.3.5 High Resolution Spectra
- 14.3.6 MS Practice Problems
- 14.3.6a Problem 1
- 14.3.6b Problem 2
- 14.3.6c Problem 3
- 14.3.6d Problem 4
- 14.3.6e Problem 5
- 14.3.6f Problem 6
- 14.3.6g Problem 7
- 14.3.6h Problem 8
- Chapter 15: Nuclear Magnetic Resonance Spectroscopy and UV-Visible SpectroscopyToggle Dropdown
- 15.1 Nuclear Magnetic Resonance Spectroscopy
- 15.1.1 Background
- 15.1.2 Proton NMR Spectroscopy
- 15.1.2a Introduction to Proton NMR Spectroscopy
- 15.1.2b Chemical Shift
- 15.1.2c Signal Strength
- 15.1.2d Hydroxyl Proton Exchange and the Influence of Hydrogen Bonding
- 15.1.2e Pi-Electron Functions
- 15.1.2f Solvent Effects
- 15.1.2g Spin-Spin Interactions
- 15.1.2h Examples
- 15.1.3 Carbon NMR Spectroscopy
- 15.1.4 NMR Practice Problems
- 15.1.4a Problem 1
- 15.1.4b Problem 2
- 15.1.4c Problem 3
- 15.1.4d Problem 4
- 15.1.4e Problem 5
- 15.1.4f Problem 6
- 15.1.4g Problem 7
- 15.1.4h Problem 8
- 15.1.4i Problem 9
- 15.1.4j Problem 10
- 15.1.5 Table of Proton NMR Shifts
- 15.1.6 Table of Carbon NMR Shifts
- 15.2 UV-Visible Spectroscopy
- 15.2.1 Background
- 15.2.2 The Electromagnetic Spectrum
- 15.2.3 UV-Visible Absorption Spectra
- 15.2.4 The Importance of Conjugation
- 15.3 Spectroscopy Practice Problems
- Chapter 16: Conjugated Pi Systems and Pericyclic ReactionsToggle Dropdown
- Chapter 17: Aromatic CompoundsToggle Dropdown
- 17.1 Aromaticity
- 17.1.1 Benzene
- 17.1.2 Fused Ring Compounds
- 17.1.3 Other Aromatic Compounds
- 17.1.4 Antiaromaticity
- 17.1.5 Practice Problems
- 17.1.5a Problem 1
- 17.1.5b Problem 2
- 17.2 Reactions of Substituent Groups
- 17.2.1 Oxidation of Alkyl Side-Chains
- 17.2.2 Bromination of Alkyl Side-Chains
- 17.2.3 Reduction of Nitro Groups
- Chapter 17 Videos
- Chapter 18: Aromatic Substitution ReactionsToggle Dropdown
- 18.1 Electrophilic Aromatic Substitution Reactions
- 18.2 Electrophilic Aromatic Substitution Mechanism
- 18.3 Electrophilic Aromatic Substitution Activation/Deactivation and Orientation
- 18.4 Electrophilic Substitution of Disubstituted Benzene Rings
- 18.5 Practice Problems
- 18.5.1 Problem 1
- 18.5.2 Problem 2
- 18.5.3 Problem 3
- 18.5.4 Problem 4
- 18.5.5 Problem 5
- 18.5.6 Problem 6
- 18.5.7 Problem 7
- Chapter 18 Videos
- Chapter 19: Aldehydes and KetonesToggle Dropdown
- 19.1 Nomenclature
- 19.2 Preparation of Aldehydes and Ketones
- 19.3 Properties of Aldehydes and Ketones
- 19.4 Reactions of Aldehydes and Ketones
- 19.4.1 Addition Reactions
- 19.4.1a Hydration
- 19.4.1b Acetal Formation
- 19.4.1c Imine Formation
- 19.4.1d Cyanohydrin Formation
- 19.4.1e Hydride Reduction
- 19.4.1f Addition of Organometallic Reagents
- 19.4.2 Reduction of Aldehydes and Ketones
- 19.4.2a Wolff-Kishner Reduction
- 19.4.2b Clemmensen Reduction
- 19.4.3 Oxidation of Aldehydes and Ketones
- 19.5 Practice Problems
- 19.5.1 Problem 1
- 19.5.2 Problem 2
- 19.5.3 Problem 3
- 19.5.4 Problem 4
- 19.5.5 Problem 5
- 19.5.6 Problem 6
- 19.5.7 Problem 7
- 19.5.8 Problem 8
- 19.5.9 Problem 9
- 19.5.10 Problem 10
- 19.5.11 Problem 11
- 19.5.12 Problem 12
- Chapter 20: Carboxylic Acids and Their DerivativesToggle Dropdown
- 20.1 Nomenclature
- 20.2 Physical Properties
- 20.3 Acidity
- 20.4 Preparation of Carboxylic Acids
- 20.5 Reactions of Carboxylic Acids
- 20.5.1 Salt Formation
- 20.5.2 Substitution of the Hydroxyl Hydrogen
- 20.5.3 Substitution of the Hydroxyl Group
- 20.5.4 Reduction
- 20.5.5 Oxidation
- 20.6 Practice Problems-Carboxylic Acids
- 20.6.1 Nomenclature Practice-1
- 20.6.2 Nomenclature Practice-2
- 20.6.3 Acidity
- 20.6.4 Reactions of Carboxylic Acids
- 20.7 Carboxylic Acid Derivatives
- 20.7.1 Related Derivatives
- 20.7.2 Nomenclature
- 20.7.3 Reactions
- 20.7.3a Acyl Substitution
- 20.7.3b Nitrile Hydrolysis
- 20.7.3c Reductions
- 20.7.3d Reactions with Organometallic Reagents
- 20.7.3e Dehydration of Amides
- 20.7.4 Practice Problems-Carboxylic Acid Derivatives
- 20.7.4a Nomenclature Practice-1
- 20.7.4b Nomenclature Practice-2
- 20.7.4c Carbonyl Compounds
- 20.8 Practice Problems
- 20.8.1 Problem 1
- 20.8.2 Problem 2
- 20.8.3 Problem 3
- 20.8.4 Problem 4
- 20.8.5 Problem 5
- 20.8.6 Problem 6
- Chapter 21: Alpha Carbon Chemistry: Enols and EnolatesToggle Dropdown
- 21.1 Reactions at the Alpha Carbon
- 21.2 Alpha Halogenation of Enols and Enolates
- 21.3 Aldol Reaction
- 21.4 Claisen Condensation
- 21.5 Alkylation at the Alpha Position
- 21.5.1 Enolate Alkylation
- 21.5.2 Dicarbonyl Alkylation
- 21.5.3 Decarboxylation Following Alkylation
- 21.5.4 Conjugate Reactions
- 21.5.4a Michael Reaction
- 21.5.4b Robinson Annulation
- 21.5.4c With Hydrides and Organometallics
- 21.6 Practice Problem
- 21.6.1 Problem 1
- Org Chem II - Problem Sets - Collection (CHEM 3402)
- Problem Set
4.3 Conformational Stereoisomers
Conformational Stereoisomers
Structural formulas show the manner in which the atoms of a molecule are bonded together (its constitution), but do not generally describe the three-dimensional shape of a molecule, unless special bonding notations (e.g. wedge and hatched lines) are used. The importance of such three-dimensional descriptive formulas became clear in discussing configurational stereoisomerism, where the relative orientation of atoms in space is fixed by a molecule's bonding constitution (e.g. double-bonds and rings). Here too it was noted that nomenclature prefixes must be used when naming specific stereoisomers. In this section we shall extend our three-dimensional view of molecular structure to include compounds that normally assume an array of equilibrating three-dimensional spatial orientations, which together characterize the same isolable compound. We call these different spatial orientations of the atoms of a molecule that result from rotations or twisting about single bonds conformations.
In the case of hexane, we have an unbranched chain of six carbons which is often written as a linear formula: CH3CH2CH2CH2CH2CH3. We know this is not strictly true, since the carbon atoms all have a tetrahedral configuration. The actual shape of the extended chain is therefore zig-zag in nature. However, there is facile rotation about the carbon-carbon bonds, and the six-carbon chain easily coils up to assume a rather different shape. Many conformations of hexane are possible and two are illustrated below.
| Extended Chain | Coiled Chain |
|---|---|
 |
 |
For an animation of conformational motion in hexane .
Ethane Conformations
The simple alkane ethane provides a good introduction to conformational analysis. Here there is only one carbon-carbon bond, and the rotational structures (rotamers) that it may assume fall between two extremes, staggered and eclipsed. In the following description of these conformers, several structural notations are used. The first views the ethane molecule from the side, with the carbon-carbon bond being horizontal to the viewer. The hydrogens are then located in the surrounding space by wedge (in front of the plane) and hatched (behind the plane) bonds. If this structure is rotated so that carbon #1 is canted down and brought closer to the viewer, the "sawhorse" projection is presented. Finally, if the viewer looks down the carbon-carbon bond with carbon #1 in front of #2, the Newman projection is seen.
|
Bond Repulsions in Ethane 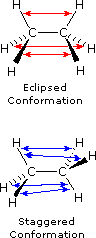 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
To see an eclipsed conformer of ethane orient itself as a Newman projection, and then interconvert with the staggered conformer and intermediate conformers .
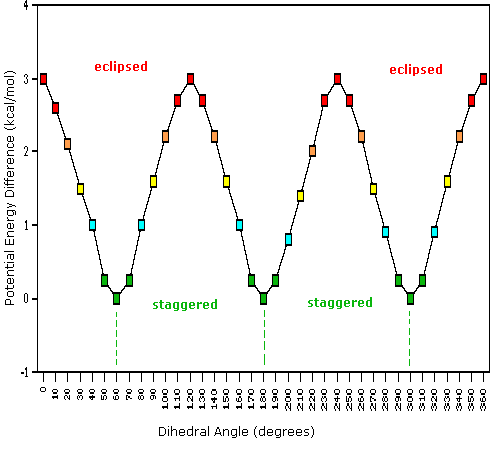
As a result of bond-electron repulsions, illustrated on the right above, the eclipsed conformation is less stable than the staggered conformation by roughly 3 kcal / mol (eclipsing strain). The most severe repulsions in the eclipsed conformation are depicted by the red arrows. There are six other less strong repulsions that are not shown. In the staggered conformation there are six equal bond repulsions, four of which are shown by the blue arrows, and these are all substantially less severe than the three strongest eclipsed repulsions. Consequently, the potential energy associated with the various conformations of ethane varies with the dihedral angle of the bonds, as shown below. Although the conformers of ethane are in rapid equilibrium with each other, the 3 kcal/mol energy difference leads to a substantial preponderance of staggered conformers (> 99.9%) at any given time.
Although steric and/or bond electron repulsion remain the most popular explanation for the hindered rotation of ethane, molecular orbital interactions have also been proposed as a significant factor. For a discussion of this feature .
Potential Energy Profile for Ethane Conformers |
Dihedral Angle | |
|---|---|---|
 |
 |
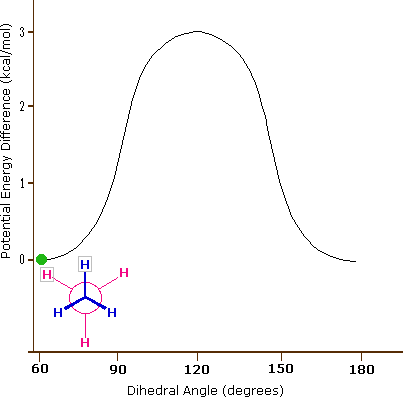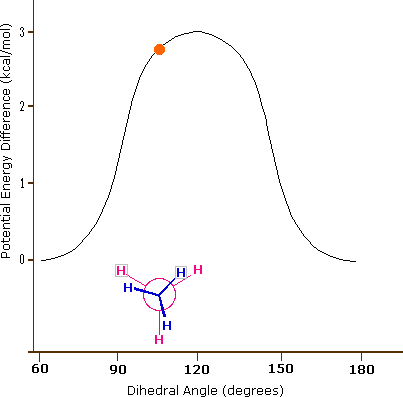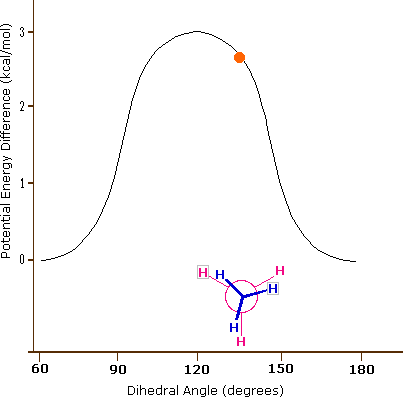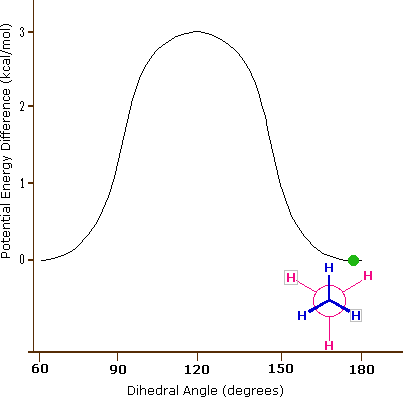 |
The above animation illustrates the relationship between ethane's potential energy and its dihedral angle |
Butane Conformations
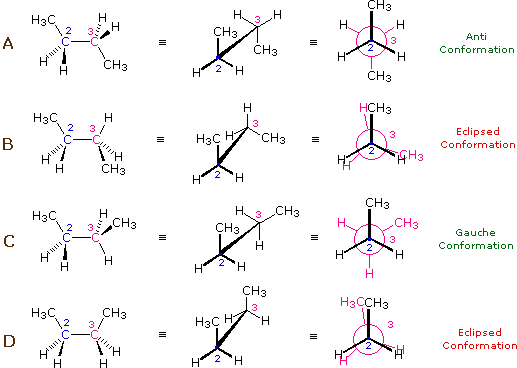
The hydrocarbon butane has a larger and more complex set of conformations associated with its constitution than does ethane. Of particular interest and importance are the conformations produced by rotation about the central carbon-carbon bond. Among these we shall focus on two staggered conformers (A & C) and two eclipsed conformers (B & D), shown below in several stereo-representations. As in the case of ethane, the staggered conformers are more stable than the eclipsed conformers by 2.8 to 4.5 kcal/mol. Since the staggered conformers represent the chief components of a butane sample they have been given the identifying prefix designations anti for A and gauche for C.
| Four Conformers of Butane |
|---|
 |
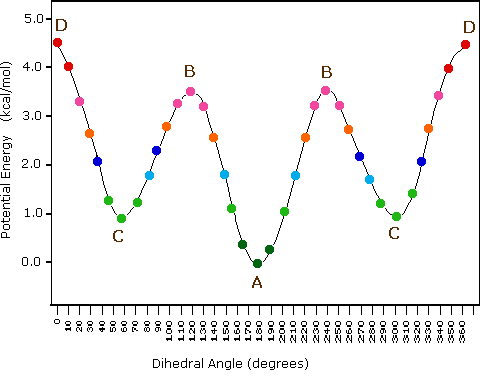
The following diagram illustrates the change in potential energy that occurs with rotation about the C2–C3 bond. The model on the right is shown in conformation D, and by clicking on any of the colored data points on the potential energy curve, it will change to the conformer corresponding to that point. The full rotation will be displayed by turning the animation on. This model may be manipulated by click-dragging the mouse for viewing from any perspective.
| Potential Energy Profile for Butane Conformers | |
|---|---|
 |
|
|
Stick Model Animation on/off |
It is useful to summarize some important aspects of conformational stereoisomerism at this time.
(i) Most conformational interconversions in simple molecules occur rapidly at room temperature. Consequently, isolation of pure conformers is usually not possible.
(ii) Specific conformers require special nomenclature terms such as staggered, eclipsed, gauche and anti when they are designated.
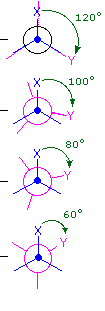
(iii) Specific conformers may also be designated by dihedral angles. In the butane conformers shown above, the dihedral angles formed by the two methyl groups about the central double bond are: A 180º, B 120º, C 60º & D 0º.
(iv) Staggered conformations about carbon-carbon single bonds are more stable (have a lower potential energy) than the corresponding eclipsed conformations. The higher energy of eclipsed bonds is known as eclipsing strain.
(v) In butane the gauche-conformer is less stable than the anti-conformer by about 0.9 kcal/mol. This is due to a crowding of the two methyl groups in the gauche structure, and is called steric strain or steric hindrance.
(vi) Butane conformers B and C have non-identical mirror image structures in which the clockwise dihedral angles are 300º & 240º respectively. These pairs are energetically the same, and have not been distinguished in the potential energy diagram shown here.

