Chemistry Textbook
- 1. Matter and MeasurementsToggle Dropdown
- 2. Atoms, Molecules and IonsToggle Dropdown
- 3. Composition of Substances and SolutionsToggle Dropdown
- 4. Stoichiometry of Chemical ReactionsToggle Dropdown
- 5. Thermochemistry
- 6. GasesToggle Dropdown
- 7. Chemical Bonding and Molecular GeometryToggle Dropdown
- 8. Liquids, Solids, and Modern MaterialsToggle Dropdown
- 9. Solutions and Colligative PropertiesToggle Dropdown
- 10. KineticsToggle Dropdown
- 11. Chemical Equilibria and ApplicationsToggle Dropdown
- 12. ThermodynamicsToggle Dropdown
- 13. ElectrochemistryToggle Dropdown
- 14. AppendicesToggle Dropdown
- Appendix A: Periodic Table of Elements
- Appendix B: Essential Mathematics
- Appendix C: Units and Conversion Factors
- Appendix D: Fundamental Physical Constants
- Appendix E: Water Properties
- Appendix F: Composition of Commercial Acids and Bases
- Appendix G: Standard Thermodynamic Properties for Selected Substances
- Appendix H: Ionization Constants of Weak Acids
- Appendix I: Ionization Constants of Weak Bases
- Appendix J: Solubility Products
- Appendix K: Formation Constants for Complex Ions
- Appendix L: Standard Electrode (Half-Cell) Potentials
Calorimetry
By the end of this section, you will be able to:
- Explain the technique of calorimetry
- Calculate and interpret heat and related properties using typical calorimetry data
Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature. When an endothermic reaction occurs, the heat required is absorbed from the thermal energy of the solution, which decreases its temperature (Figure 5.7). The temperature change, along with the specific heat and mass of the solution, can then be used to calculate the amount of heat involved in either case.
Constant Pressure Calorimetry (Coffee Cup Calorimeter)
Scientists use well-insulated calorimeters that all but prevent the transfer of heat between the calorimeter and its environment, which effectively limits the “surroundings” to the nonsystem components with the calorimeter (and the calorimeter itself). This enables the accurate determination of the heat involved in chemical processes, the energy content of foods, and so on. General chemistry students often use simple calorimeters constructed from polystyrene cups (Figure 5.8). These easy-to-use “coffee cup” calorimeters allow more heat exchange with the outside environment, and therefore produce less accurate energy values.

Figure 5.8 A simple calorimeter can be constructed from two polystyrene cups. A thermometer and stirrer extend through the cover into the reaction mixture.
Suppose we initially have a high-temperature substance, such as a hot piece of metal (M), and a low-temperature substance, such as cool water (W). If we place the metal in the water, heat will flow from M to W. The temperature of M will decrease, and the temperature of W will increase, until the two substances have the same temperature—that is, when they reach thermal equilibrium. If this occurs in a calorimeter, ideally all of this heat transfer occurs between the two substances, with no heat gained or lost by either its external environment. Under these ideal circumstances, the net heat change is zero:
This relationship can be rearranged to show that the heat gained by substance M is equal to the heat lost by substance W:
The magnitude of the heat (change) is therefore the same for both substances, and the negative sign merely shows that qsubstance M and qsubstance W are opposite in direction of heat flow (gain or loss) but does not indicate the arithmetic sign of either q value (that is determined by whether the matter in question gains or loses heat, per definition). In the specific situation described, qsubstance M is a negative value and qsubstance W is positive, since heat is transferred from M to W.
This method can also be used to determine other quantities, such as the specific heat of an unknown metal.
When we use calorimetry to determine the heat involved in a chemical reaction, the same principles we have been discussing apply. The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as discussed later), and the calorimeter minimizes energy exchange with the outside environment. Because energy is neither created nor destroyed during a chemical reaction, the heat produced or consumed in the reaction (the “system”), qreaction, plus the heat absorbed or lost by the solution (the “surroundings”), qsolution, must add up to zero:
This means that the amount of heat produced or consumed in the reaction equals the amount of heat absorbed or lost by the solution:
This concept lies at the heart of all calorimetry problems and calculations.
Constant Volume Calorimetry (Bomb Calorimeter)
Usually a coffee-cup calorimeter is used since it is simpler than a bomb calorimeter, but to measure the heat evolved in a combustion reaction, constant volume or bomb calorimetry is ideal. A constant volume calorimeter is also more accurate than a coffee-cup calorimeter, but it is more difficult to use since it requires a well-built reaction container that is able to withstand large amounts of pressure changes that happen in many chemical reactions (Figure 5.9).
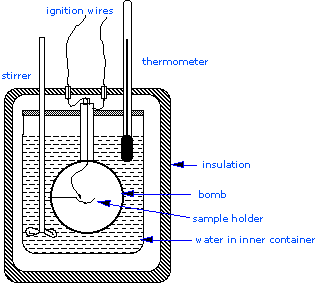
Most serious calorimetry carried out in research laboratories involves the determination of heats of combustion (ΔHcombustion) since these are essential to the determination of standard enthalpies of formation of the thousands of new compounds that are prepared and characterized each month. In a constant volume calorimeter, the system is sealed or isolated from its surroundings, which accounts for why its volume is fixed and there is no volume-pressure work done. A bomb calorimeter structure consists of the following:
- Steel bomb which contains the reactants
- Water bath in which the bomb is submerged
- Thermometer
- A motorized stirrer
- Wire for ignition
Since the process takes place at constant volume, the reaction vessel must be constructed to withstand the high pressure resulting from the combustion process, which amounts to a confined explosion. The vessel is usually called a “bomb”, and the technique is known as bomb calorimetry. The reaction is initiated by discharging a capacitor through a thin wire which ignites the mixture.
A 360.0-g piece of rebar (a steel rod used for reinforcing concrete) is dropped into 425 mL of water at 24.0 °C. The final temperature of the water was measured as 42.7 °C. Calculate the initial temperature of the piece of rebar. Assume the specific heat of steel is approximately the same as that for iron (Table 5.1), and that all heat transfer occurs between the rebar and the water (there is no heat exchange with the surroundings).
The temperature of the water increases from 24.0 °C to 42.7 °C, so the water absorbs heat. That heat came from the piece of rebar, which initially was at a higher temperature. Assuming that all heat transfer was between the rebar and the water, with no heat “lost” to the outside environment, then heat given off by rebar = −heat taken in by water, or:
Since we know how heat is related to other measurable quantities, we have:
Letting f = final and i = initial, in expanded form, this becomes:
The density of water is 1.0 g/mL, so 425 mL of water = 425 g. Noting that the final temperature of both the rebar and water is 42.7 °C, substituting known values yields:
Solving this gives Ti,rebar= 248 °C, so the initial temperature of the rebar was 248 °C.
A 248-g piece of copper is dropped into 390 mL of water at 22.6 °C. The final temperature of the water was measured as 39.9 °C. Calculate the initial temperature of the piece of copper. Assume that all heat transfer occurs between the copper and the water.
The initial temperature of the copper was 335.6 °C.
A 59.7 g piece of metal that had been submerged in boiling water was quickly transferred into 60.0 mL of water initially at 22.0 °C. The final temperature is 28.5 °C. Use these data to determine the specific heat of the metal. Use this result to identify the metal.
Solution:
Assuming perfect heat transfer, heat given off by metal = −heat taken in by water, or:
In expanded form, this is:
Noting that since the metal was submerged in boiling water, its initial temperature was 100.0 °C; and that for water, 60.0 mL = 60.0 g; we have:
Solving this:
Comparing this with values in Table 5.1, our experimental specific heat is closest to the value for copper (0.39 J/g °C), so we identify the metal as copper.
Check Your Learning:
A 92.9-g piece of a silver/gray metal is heated to 178.0 °C, and then quickly transferred into 75.0 mL of water initially at 24.0 °C. After 5 minutes, both the metal and the water have reached the same temperature: 29.7 °C. Determine the specific heat and the identity of the metal. (Note: You should find that the specific heat is close to that of two different metals. Explain how you can confidently determine the identity of the metal).
cmetal= 0.13 J/g °C
This specific heat is close to that of either gold or lead. It would be difficult to determine which metal this was based solely on the numerical values. However, the observation that the metal is silver/gray in addition to the value for the specific heat indicates that the metal is lead.
When 50.0 mL of 1.00 M HCl(aq) and 50.0 mL of 0.990 M NaOH(aq), both at 22.0 °C, are added to a coffee cup calorimeter, the temperature of the mixture reaches a maximum of 28.9 °C. What is the approximate amount of heat produced by this reaction?
Solution:
To visualize what is going on, imagine that you could combine the two solutions so quickly that no reaction took place while they mixed; then after mixing, the reaction took place. At the instant of mixing, you have 100.0 mL of a mixture of HCl and NaOH at 22.0 °C. The HCl and NaOH then react until the solution temperature reaches 28.9 °C.
The heat given off by the reaction is equal to that taken in by the solution. Therefore:
(It is important to remember that this relationship only holds if the calorimeter does not absorb any heat from the reaction, and there is no heat exchange between the calorimeter and the outside environment.)
Next, we know that the heat absorbed by the solution depends on its specific heat, mass, and temperature change:
To proceed with this calculation, we need to make a few more reasonable assumptions or approximations. Since the solution is aqueous, we can proceed as if it were water in terms of its specific heat and mass values. The density of water is approximately 1.0 g/mL, so 100.0 mL has a mass of about 1.0 102 g (two significant figures). The specific heat of water is approximately 4.184 J/g °C, so we use that for the specific heat of the solution. Substituting these values gives:
Finally, since we are trying to find the heat of the reaction , we have:
The negative sign indicates that the reaction is exothermic. It produces 2.9 kJ of heat.
Since ΔH=q at constant pressure, we can also say that ΔH = -2.9 kJ when 50 mL each of 1.00 M HCl and 1.00 M NaOH reacted.
Calculating heat transferred per mole of reaction:
First, let's analyze how many moles of HCl (or NaOH) reacted in the calorimeter.
Since ratio of moles of HCl and NaOH (0.0500/0.0495) is greater than the Stoichiometric ratio (1:1 from balanced equation), HCl is the excess reagent and NaOH is the limiting reagent. So, during the reaction, all 0.0495 mol of NaOH reacted (which also consumed 0.0495 mol of HCl).
Therefore, the ΔH or qreaction=-2.9kJ that we calculated is the heat of the reaction when 0.0495 mol of NaOH reacted.
Heat of reaction per mol NaOH is calculated as:
which is also -59 kJ/mol reaction as seen from the molar ratio,1:1, between NaOH and the reaction:
Check Your Learning:
When 100 mL of 0.200 M NaCl(aq) and 100 mL of 0.200 M AgNO3(aq), both at 21.9 °C, are mixed in a coffee cup calorimeter, the temperature increases to 23.5 °C as solid AgCl forms. Is the reaction exothermic or endothermic? How much heat (in kJ/mol) is transferred in the precipitation reaction? What assumptions did you make to determine your value?
The solution's temperature has increased. This means the solution absorbed heat from the reaction (ie. the reaction released heat). Therefore, the reaction is exothermic. ΔH or qreaction= -67 kJ/mol; assume no heat is absorbed by the calorimeter, no heat is exchanged between the calorimeter and its surroundings, and that the specific heat and density of the solution are the same as those for water
