Chemistry Textbook
- 1. Matter and Measurements Toggle Dropdown
- 2. Atoms, Molecules and Ions Toggle Dropdown
- 3. Composition of Substances and Solutions Toggle Dropdown
- 4. Stoichiometry of Chemical Reactions Toggle Dropdown
- 5. Thermochemistry Toggle Dropdown
- 6. Gases Toggle Dropdown
- 7. Chemical Bonding and Molecular Geometry Toggle Dropdown
- 8. Liquids, Solids, and Modern Materials
- 9. Solutions and Colligative Properties Toggle Dropdown
- 10. Kinetics Toggle Dropdown
- 11. Chemical Equilibria and Applications Toggle Dropdown
- 12. Thermodynamics Toggle Dropdown
- 13. Electrochemistry Toggle Dropdown
-
14. Appendices
Toggle Dropdown
- Appendix A: Periodic Table of Elements
- Appendix B: Essential Mathematics
- Appendix C: Units and Conversion Factors
- Appendix D: Fundamental Physical Constants
- Appendix E: Water Properties
- Appendix F: Composition of Commercial Acids and Bases
- Appendix G: Standard Thermodynamic Properties for Selected Substances
- Appendix H: Ionization Constants of Weak Acids
- Appendix I: Ionization Constants of Weak Bases
- Appendix J: Solubility Products
- Appendix K: Formation Constants for Complex Ions
- Appendix L: Standard Electrode (Half-Cell) Potentials
Molecular Materials
By the end of this section, you will be able to:
- Define and describe polymers, both naturally occurring and synthetic polymers
- Distinguish modern materials between ceramic and composite materials
Polymers
Many of the molecular materials in consumer goods today, however, have very high molecular masses, ranging from thousands to millions of atomic mass units, and are formed from a carefully controlled series of reactions that produce giant molecules called polymers (from the Greek poly and meros, meaning “many parts”). Polymer is a giant molecule that consists of many basic structural units called monomers connected in a chain or network by covalent bonds. Polymers are used in corrective eye lenses, plastic containers, clothing and textiles, and medical implant devices, among many other uses. As shown schematically in Figure 8.27, polymerization.
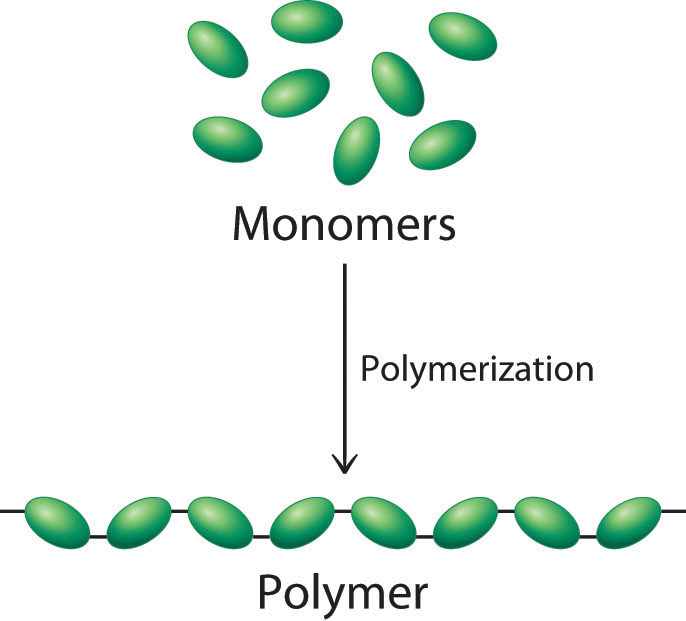
Types of Polymers: (a) Naturally Occurring and (b) Synthetic
Naturally Occurring Polymers: Peptides and Proteins
Polymers that occur naturally are crucial components of all organisms and form the fabric of our lives. Hair, silk, skin, feathers, muscle, and connective tissue are all primarily composed of proteins, the most familiar kind of naturally occurring, or biological, polymer. The monomers of many biological polymers are the amino acids each called an amino acid residue. The residues are linked together by amide bonds, also called peptide bonds, via a condensation reaction (Figure 8.28) where H2O is eliminated:
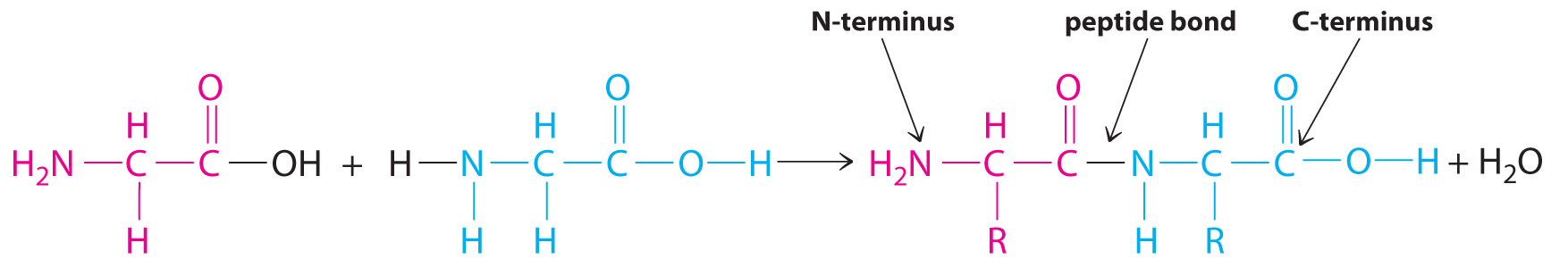
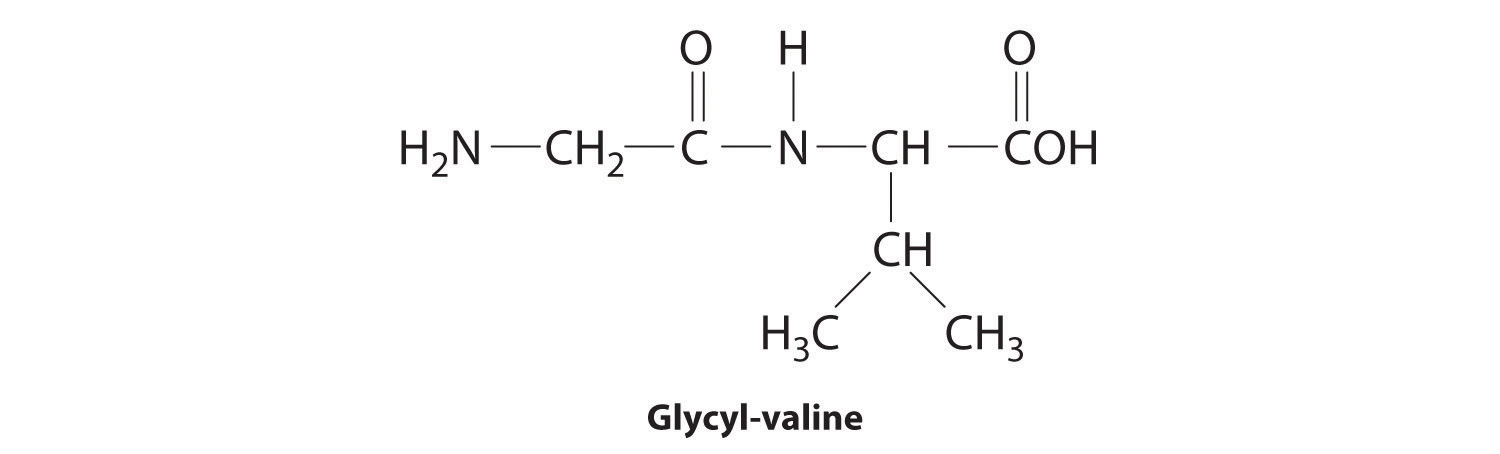
In the above equation, R represents an alkyl or aryl group, or hydrogen, depending on the amino acid. We write the structural formula of the product with the free amino group on the left (the N-terminus) and the free carboxylate group on the right (the C-terminus). For example, the structural formula for the product formed from the amino acids glycine and valine (glycyl-valine) is as follows:
Synthetic Polymers
Many of the synthetic polymers we use, such as plastics and rubbers, have commercial advantages over naturally occurring polymers because they can be produced inexpensively. Moreover, many synthetic polymers are actually more desirable than their natural counterparts because scientists can select monomer units to tailor the physical properties of the resulting polymer for particular purposes. For example, in many applications, wood has been replaced by plastics that are more durable, lighter, and easier to shape and maintain. Polymers are also increasingly used in engineering applications where weight reduction and corrosion resistance are required. Steel rods used to support concrete structures, for example, are often coated with a polymeric material when the structures are near ocean environments where steel is vulnerable to corrosion. In fact, the use of polymers in engineering applications is a very active area of research.
Probably the best-known example of a synthetic polymer is nylon . Its monomers are linked by amide bonds (which are called peptide bonds in biological polymers), so its physical properties are similar to those of some proteins because of their common structural unit—the amide group.
Video 8.4 showing synthesis of Nylon 6,10. Nylon is a synthetic condensation polymer created by the reaction of a dicarboxylic acid and a diamine to form amide bonds and water
Polyethylene is used in a wide variety of products, including beach balls and the hard plastic bottles used to store solutions in a chemistry laboratory. Which of these products is formed from the more highly branched polyethylene?
Solution:
Determine whether the polymer is LDPE, which is used in applications that require flexibility, or HDPE, which is used for its strength and rigidity.
A highly branched polymer is less dense and less rigid than a relatively unbranched polymer. Thus hard, strong polyethylene objects such as bottles are made of HDPE with relatively few branches. In contrast, a beach ball must be flexible so it can be inflated. It is therefore made of highly branched LDPE.
Check your Learning:
Which products are manufactured from LDPE and which from HPDE?
1. lawn chair frames
2. rope
3. disposable syringes
4. automobile protective covers
Answer:
- HDPE
- LDPE
- HDPE
- LDPE
Modern Materials
Modern materials are developed through the invention of new or improved processes, for example, as a result of 'man' made materials/ingredients or human intervention, in other words not naturally occurring changes. They are altered to perform a particular function. Many smart and modern materials are developed for specialised applications but some eventually become available for general use.
Ceramics
A ceramic is any nonmetallic inorganic solid that is strong enough for use in structural applications. Traditional ceramics, which are based on metal silicates or aluminosilicates, are the materials used to make pottery, china, bricks, and concrete. Modern ceramics contain a much wider range of components and can be classified as either ceramic oxides, which are based on metal oxides such as alumina (Al2O3), zirconia (ZrO2), and beryllia (BeO), or nonoxide ceramics, which are based on metal carbides such as silicon carbide (carborundum, SiC) and tungsten carbide (WC), or nitrides like silicon nitride (Si3N4) and boron nitride (BN).
https://www.sciencelearn.org.nz/videos/1060-defining-ceramics
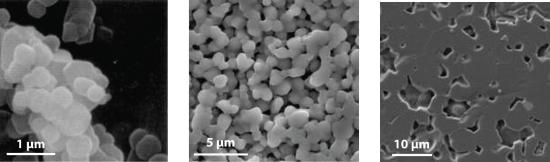
All modern ceramics are hard, lightweight, and stable at very high temperatures. Unfortunately, however, they are also rather brittle, tending to crack or break under stresses that would cause metals to bend or dent. Thus a major challenge for materials scientists is to take advantage of the desirable properties of ceramics, such as their thermal and oxidative stability, chemical inertness, and toughness, while finding ways to decrease their brittleness to use them in new applications. Few metals can be used in jet engines, for example, because most lose mechanical strength and react with oxygen at the very high operating temperatures inside the engines (approximately 2000°C). In contrast, ceramic oxides such as Al2O3 cannot react with oxygen regardless of the temperature because aluminum is already in its highest possible oxidation state (Al3+). Even nonoxide ceramics such as silicon and boron nitrides and silicon carbide are essentially unreactive in air up to about 1500°C. Producing a high-strength ceramic for service use involves a process called sintering. A process that fuses the grains of a ceramic into a dense, strong material (Figure 8.29).
Composite Materials
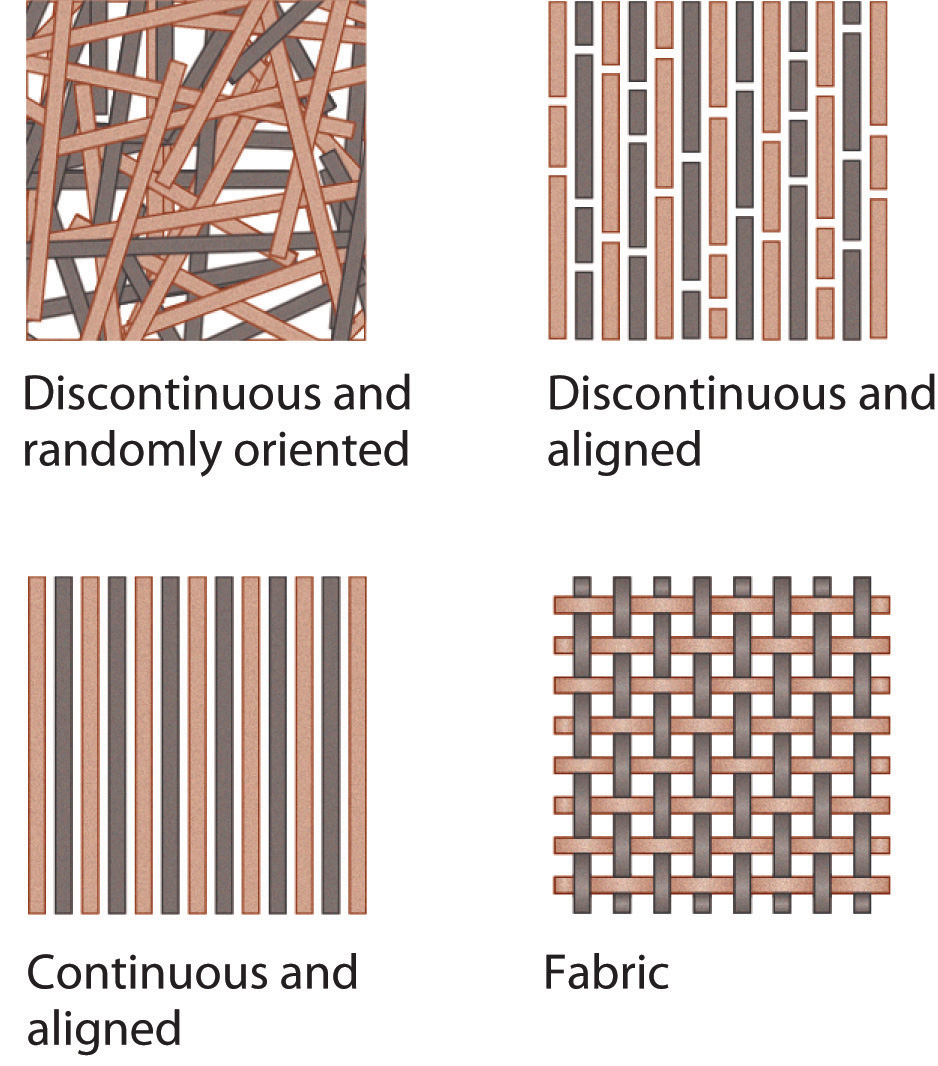
Composite materials have at least two distinct components: the matrix (which constitutes the bulk of the material) and fibers or granules that are embedded within the matrix and limit the growth of cracks by pinning defects in the bulk material (Figure 8.30). The resulting material is stronger, tougher, stiffer, and more resistant to corrosion than either component alone. Composites are thus the nanometer-scale equivalent of reinforced concrete, in which steel rods greatly increase the mechanical strength of the cement matrix, and are extensively used in the aircraft industry, among others. For example, the Boeing 777 is 9% composites by weight, whereas the newly developed Boeing 787 is 50% composites by weight. Not only does the use of composite materials reduce the weight of the aircraft, and therefore its fuel consumption, but it also allows new design concepts because composites can be molded. Moreover, by using composites in the Boeing 787 multiple functions can be integrated into a single system, such as acoustic damping, thermal regulation, and the electrical system.
Three distinct types of composite material are generally recognized, distinguished by the nature of the matrix. These are polymer-matrix composites, metal-matrix composites, and ceramic-matrix composites.
Video illustrates the properties of composite materials.